The life-span of the tooth is intimately-associated with healthiness of periodontal ligament (PDL) which is a connective tissue situated between bone and cementum that covers tooth root surface. However, once this tissue is severely damaged by deep caries, periodontitis, and trauma, this leads to severe difficulty in its regeneration, resulting in tooth loss and decreased quality of life. The development of the therapy for generation and regeneration of the periodontal tissue is an urgent issue. Therefore, researchers have tried to improve efficiently-generative and regenerative medicine using stem cells, signal molecules, and scaffolds, requisite for tissue regeneration.In recent studies, a dental follicle tissue that is composed of stem cell population potentially differentiating into PDL tissue, cementum, and alveolar bone is of current interest. More recently a revolutionary and attractive studyreporting the development of bio-hybrid implant that reserved newly-formed cementum/PDL tissue complex on its surface was introduced.In this review, we describe comprehensive reports that tried to develop the cementum/PDL complex by tissue engineering and future prospects.
bio-hybrid implant, cementum, dental follicle, periodontal ligament, periodontal generation and regeneration
The periodontal tissueis composed of four major tissues: periodontal ligament (PDL) tissue, cementum, alveolar bone, and gingival tissue.PDL tissue is composed of heterogeneous cell populations, various extracellular proteins including collagens, elastic system fibers, fibronectin, osteocalcin, proteoglycans, vitronectin, and matricellular proteins, nerve fibers, and blood vessels [1]. PDL cell population includes fibroblasts that are principal cells in PDL tissue [2,3], PDL stem cells (PDLSCs), epithelial cell rests of Malassez that are descendants of the Hertwig’s epithelial root sheath (HERS), endothelial cells, and others. Especially, PDLSCs have the potentials to differentiate into PDL fibroblasts, cementoblasts and osteoblasts [4-6]. Aged PDLSCs decrease tissue regenerative potential, but they can be restored by the extrinsic microenvironment, suggesting the retention of their plasticity [7]. Thus researchers have tried to characterize PDLSCs for generation and regeneration of the periodontal tissue in the last decade.
The central role of PDL tissue is to connect tooth root through cementum to the bone socket by forming the Sharpey’s fibers, which are composed of collagen bundles that insert their terminus into cementum and bone surfaces. This function is essentially maintained by three dimensionalstructure of collagens and elastic system fibers.Other functions involve cushioning mechanical stresses such as compressive and tensile forces arisen by mastication, and adjusting bite force depending on food hardness via sensory nerve located in the PDL tissue. Thus, it is not an exaggeration to say that PDL tissue determines the life-span of tooth.
The Cephalic neural crest (CNC) cells are principal in fabricating many mesenchymal structures during craniofacial development, including teeth, bones, muscles, and neurons [8,9]. Some reports described a hierarchy of periodontal lineage segregated from migratory CNC cells through intermediate dental follicle (DF) cells [10-12].
Dental tissue-derived mesenchymal stem cells, such as PDLSCs and dental follicle progenitor cells (DFPCs) were characterized with respect to their feasibility of PDL-like and cementum-like formations [13]. Cementogenesis is indispensable in PDL tissue formation and reformation.
Sonoyama et al. developed the ‘bio-root’ by using PDLSCs and stem cells from apical papilla with tooth root-shaped hydroxyl apatitetri calcium phosphate (HA/TCP), and showed the formation of PDL tissue on the surface of the ‘bio-root’ when transplanted in swine [14]. Although they did not show apparent cementum deposition on the HA/TCP, this group improved the ‘bio-root’ by utilizing the PDLSC sheet, showing the defined periodontal tissue structure including cementum-like formation [15].
In addition, PDLSC pellet,PDL cell sheet, PDLSCs cultured with conditioned medium from developing apical tooth germ cells have been introduced to construct cementum/PDL complex in vivo [16-18]. A recent study described the pivotal role of HERS that appears during root formation in cementogenesis as well as root formation [19].
On the other hand, multifunctionality of signal molecules in fabricating cementum/PDL tissue complex is requisite. The innatefeatures of many factors have been discovered, including activin A [20], angiotensin [21], basic fibroblast growth factor (bFGF or FGF2) [22], bone morphogenetic proteins (BMPs)[23-27], brain-derived neurotrophic factor (BDNF) [28], calcium [29], connective tissue growth factor (CTGF/CCN2) [30,31], enamel matrix derivative (EMD) [32,33], epidermal growth factor (EGF) [34,35], glial cell-line derived neurotrophic factor (GDNF) [36], growth/differentiation factor-5 (GDF-5/BMP-14) [37], insulin-like growth factor-1 (IGF-1) [27,38], interleukin-11 [39], platelet-derived growth factor (PDGF) [40,41], transforming growth factor-β (TGF-β) [1,42-44], and BMP-2+ nerve growth factor (NGF) [45]. Researchers need to exploit properties of these factors in a single or combined use for periodontal generation and regeneration.
As scaffolds for periodontal construction and reconstruction, two types have been used: synthetic and natural materials that include degradable or nondegradable properties [46]. As synthetic degradable materials, polyethylene glycol, poly-lactic acid (PLA), poly-Llactic acid, poly-glycolic acid, poly(lactic-co-glycolic acid), poly(L-lactide-co-D,L-lactide), polycaprolactone, polyvinyl alcohol, poly(2-hydroxyethyl methacrylate) (PHEMA), and polymethylmethacrylate (PMMA) and PHEMA composites have been studied, while PMMA, polytetrafluoroethylene, and 4-methacryloxyethyl trimellitate anhydride/methyl methacrylate (4-META/MMA) have been advanced as synthetic nondegradable materials. On the other hand, as natural materials, alginate, agarose, chitosan, collagen, atelocollagen, gelatin, fibrin, and hyaluronic acid have been examined. In addition, Ceramics such as hydroxyapatite and β–TCP have also been investigated about their potentials for tissue generation.
DF is a mesenchymal tissue sac surrounding tooth germ and appears only during tooth development [47]. DF cells show self-renewal and multipotency to differentiate into osteoblasts, adipocytes, and neurocytes in vitro [48,49]. In addition, DF reportedly possesses various intermediate progenitors that differentiate into PDL fibroblasts, cementoblasts, and osteoblasts [11,12]. Therefore, researchers have pursued potentials of these cells in generating and regenerating cementum/PDL complex.
Kemoun et al. reported cementoblastic differentiation of human DF cells cultured with enamel matrix derivatives [50]. Guo et al. demonstrated thecementum/PDL construction by rat DF cells on the surface of EDTA-treated dentin matrix in vivo using a rat model [51]. Recently an interesting study was reported, showing that when rat adipose tissue-deprived stem cells (ADSCs) were cultured in DF cell-conditioned medium supplemented with an inhibitor of the Wnt pathway, ADSCs differentiated into cementoblast-like cells, suggesting contribution of Wnt/-&beta -catenin pathway in cementogenesis [52]. By contrast, another study showed that HERS regulates osteogenic differentiation of DF cells through the Wnt pathway, and that Wnt3a enhanced the differentiation of DF cells [53]. Thus the roles of the Wnt signal pathway in constructing cementum/PDL complex remain to be solved.
In this context, Oshima et al. reported the revolutionary study concerning the development of the ‘bio-hybrid dental implant’ that is a hydroxyapatite-coated titanium covered by murine DF tissue at ED 18.5, which was detached from developing tooth germ [54]. They demonstrated the development of functional cementum/PDL tissue complex including the formation of the Sharpey-fibers, and innervation around this implant, which allowed to response to orthodontic force, and to restore vertically lost bone defect, indicating features very similar to natural tooth.
The longevity rate in many countries of the world is getting longer. In such circumstances, to avoid tooth loss and to retain ones’ own teeth as many and long as possible lead to maintaining their quality of life. Of course, replacement therapy with osseo-integrated implants is current mainstream, but a statistical study performed in the USA showed that many people desire to keep and use their own teeth during their lifetime even though patientswho experienced successful implant treatment [55]. As described above, a report by Oshima et al. opened the way for generation and regeneration of periodontal tissueby using DF cells from tooth germ at temporally-limited stage of development, while it was restricted to murine specimen [54]. Therefore considering clinical practice in patients for structuring human periodontal tissue with much larger size, researchers need to clarify the characteristics of DF cells at 18.5, comparing with those at other stages or PDLSCs, and how they can obtain such DF-like cells from bone marrow mesenchymal stem cells (BMMSCs), induced pluripotent stem (iPS) cells or other mesenchymal stem cell sources(MSCs) because the quantity of PDLSCs localized in PDL tissue is too small to use in clinical use while PDLSCs are naturally eligible (Figure 1). In addition,which bioactive scaffolds and multifunctional signal molecules support the differentiation of these stem cells also remains to be determined.Sufficient assessment of outcomes from integration of these elements from various aspectsis needed for restoration ofperiodontal function.
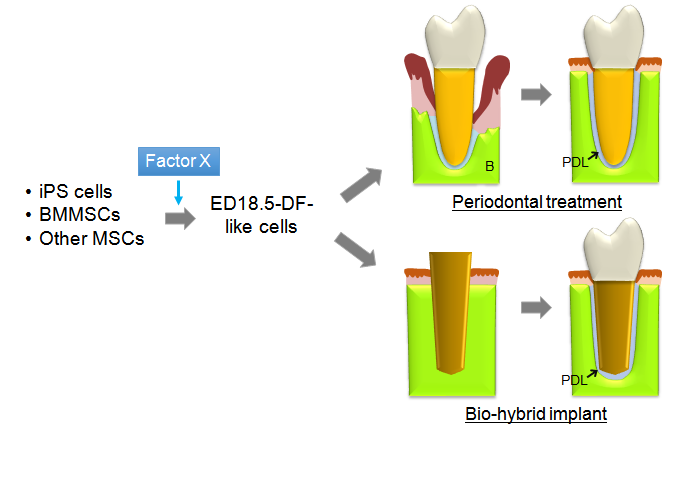
Figure 1. Schema representing the next-generative therapy by tissue engineering using induced DF-like cells. iPS cells, BMMSCs, and other MSCs could be directed toward DF-like cells by factor X that is needed to be identified by characterizing DF cells at ED 18.5. The development of this novel and promising therapy will permit generation and regeneration of periodontal tissue. B, Bone; PDL, Periodontal ligament.
The authors declare that they have no competing interests.
- 1. Maeda H, Wada N, Tomokiyo A, Monnouchi S, Akamine A (2013) Prospective potency of TGF-beta1 on maintenance and regeneration of periodontal tissue. Int Rev Cell Mol Biol 304: 283-367. [Crossref]
- 2. Beertsen W, McCulloch CA, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13: 20-40. [Crossref]
- 3. Berkovitz BKB, Shore RC (1995) Cells of periodontal ligament. Mosby-Wolfe, London.
- 4. Dangaria SJ, Ito Y, Luan X, Di/ekwisch TG (2011) Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem Cells Dev 20: 1659-1668. [Crossref]
- 5. Maeda H, Fujii S, Tomokiyo A, Wada N, Akamine A (2013) Periodontal tissue engineering: defining the triad. Int J Oral Maxillofac Implants 28: e461-471. [Crossref]
- 6. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149-155. [Crossref]
- 7. Zheng W, Wang S, Ma D, Tang L, Duan Y, et al. (2009) Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng Part A 15: 2363-71. [Crossref]
- 8. Couly G, Le Douarin NM (1990) Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development 108: 543-558. [Crossref]
- 9. Dupin E, Calloni GW, Le Douarin NM (2010) The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities. Cell Cycle 9: 238-49. [Crossref]
- 10. Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, et al. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127: 1671-1679. [Crossref]
- 11. Luan X, Dangaria S, Ito Y, Walker CG, Jin T, et al. (2009) Neural crest lineage segregation: a blueprint for periodontal regeneration. J Dent Res 88: 781-791. [Crossref]
- 12. Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, et al. (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24: 155-165. [Crossref]
- 13. Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88: 792-806. [Crossref]
- 14. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, et al. (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1: e79. [Crossref]
- 15. Wei F, Song T, Ding G, Xu J, Liu Y, et al. (2013) Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev 22: 1752-1762. [Crossref]
- 16. Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, et al. (2008) Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res 43: 364-371. [Crossref]
- 17. Yang Z, Jin F, Zhang X, Ma D, Han C (2009) Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for human periodontal ligament stem cells. Tissue Eng Part C Methods 15: 571-81. [Crossref]
- 18. Yang ZH, Zhang XJ, Dang NN, Ma ZF, Xu L, et al. (2009) Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res 44: 199-210. [Crossref]
- 19. Xiong J, Gronthos S, Bartold PM (2013) Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol 2000 63: 217-233. [Crossref]
- 20. Sugii H, Maeda H, Tomokiyo A, Yamamoto N, Wada N, et al. (2014) Effects of Activin A on the phenotypic properties of human periodontal ligament cells. Bone 66: 62-71. [Crossref]
- 21. Monnouchi S, Maeda H, Fujii S, Tomokiyo A, Kono K, et al. (2011) The roles of angiotensin II in stretched periodontal ligament cells. J Dent Res 90: 181-185. [Crossref]
- 22. Murakami S (2011) Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000 56: 188-208. [Crossref]
- 23. Chiu HC, Chiang CY, Tu HP, Wikesjö UM, Susin C, et al. (2013) Effects of bone morphogenetic protein-6 on periodontal wound healing/regeneration in supraalveolar periodontal defects in dogs. J Clin Periodontol 40: 624-630. [Crossref]
- 24. Hakki SS, Bozkurt B, Hakki EE, Kayis SA, Turac G, et al. (2014) Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J Biomed Mater Res B Appl Biomater 102: 119-130. [Crossref]
- 25. King GN, King N, Cruchley AT, Wozney JM, Hughes FJ (1997) Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J Dent Res 76: 1460-1470. [Crossref]
- 26. Sorensen RG, Polimeni G, Kinoshita A, Wozney JM, Wikesjö UM (2004) Effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of periodontal attachment following tooth replantation in dogs. J Clin Periodontol 31: 654-661. [Crossref]
- 27. Yang L, Zhang Y, Dong R, Peng L, Liu X, et al. (2010) Effects of adenoviral-mediated coexpression of bone morphogenetic protein-7 and insulin-like growth factor-1 on human periodontal ligament cells. J Periodontal Res 45: 532-40. [Crossref]
2021 Copyright OAT. All rights reserv
- 28. Takeda K, Shiba H, Mizuno N, Hasegawa N, Mouri Y, et al. (2005) Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng 11: 1618-1629. [Crossref]
- 29. Koori K, Maeda H, Fujii S, Tomokiyo A, Kawachi G, et al. (2014) The roles of calcium-sensing receptor and calcium channel in osteogenic differentiation of undifferentiated periodontal ligament cells. Cell Tissue Res. 357: 707-18. [Crossref]
- 30. Asano M, Kubota S, Nakanishi T, Nishida T, Yamaai T, et al. (2005) Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells. Cell Commun Signal 3:11. [Crossref]
- 31. Yuda A, Maeda H, Fujii S, Monnouchi S, Yamamoto N, et al. (2014) Effect of CTGF/CCN2 on osteo/cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line. J Cell Physiol. [Crossref]
- 32. Grandin HM, Gemperli AC, Dard M (2012) Enamel matrix derivative: a review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng Part B Rev 18: 181-202. [Crossref]
- 33. Zetterström O, Andersson C, Eriksson L, Fredriksson A, Friskopp J, et al. (1997) Clinical safety of enamel matrix derivative (EMDOGAIN) in the treatment of periodontal defects. J Clin Periodontol 24: 697-704. [Crossref]
- 34. Nishimura F, Terranova VP (1996) Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J Dent Res 75: 986-992. [Crossref]
- 35. Teramatsu Y, Maeda H, Sugii H, Tomokiyo A, Hamano S, et al. (2014) Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell Tissue Res 357: 633-643. [Crossref]
- 36. Yamamoto N, Maeda H, Tomokiyo A, Fujii S, Wada N, et al. (2012) Expression and effects of glial cell line-derived neurotrophic factor on periodontal ligament cells. J Clin Periodontol 39: 556-564. [Crossref]
- 37. Kwon HR, Wikesjö UM, Park JC, Kim YT, Bastone P(2010) Growth/differentiation factor-5 significantly enhances periodontal wound healing/regeneration compared with platelet-derived growth factor-BB in dogs. J Clin Periodontol. 37: 739-46. [Crossref]
- 38. Chen FM, Zhao YM, Wu H, Deng ZH, Wang QT, et al. (2006) Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release 114: 209-222. [Crossref]
- 39. Monnouchi S, Maeda H, Yuda A, Hamano S, Wada N, et al.(2014) Mechanical induction of interleukin-11 regulates osteoblastic/cementoblastic differentiation of human periodontal ligament stem/progenitor cells. J Periodontal Res. [Crossref]
- 40. Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, et al. (1989) A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol 16: 545-548. [Crossref]
- 41. Thakare K, Bhongade ML, Charde P, Jaiswal P, Shah N, et al. (2013) Periodontal regeneration using platelet-derived growth factor in infrabony defects: a series of three cases. Case Rep Dent 2013: 849823. [Crossref]
- 42. Fujii S, Maeda H, Tomokiyo A, Monnouchi S, Hori K, et al. (2010) Effects of TGF-β1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res 342: 233-242. [Crossref]
- 43. Teare JA, Ramoshebi LN, Ripamonti U (2008) Periodontal tissue regeneration by recombinant human transforming growth factor-beta 3 in Papioursinus. J Periodontal Res 43: 1-8. [Crossref]
- 44. Wikesjö UM, Razi SS, Sigurdsson TJ, Tatakis DN, Lee MB, et al. (1998) Periodontal repair in dogs: effect of recombinant human transforming growth factor-beta1 on guided tissue regeneration. J Clin Periodontol 25: 475-481. [Crossref]
- 45. Yan XZ, Ge SH, Sun QF, Guo HM, Yang PS (2010) A pilot study evaluating the effect of recombinant human bone morphogenetic protein-2 and recombinant human beta-nerve growth factor on the healing of Class III furcation defects in dogs. J Periodontol 81: 1289-1298. [Crossref]
- 46. Maeda H, Tomokiyo A, Wada N, Koori K, Kawachi G, et al. (2014) Regeneration of the periodontium for preservation of the damaged tooth. Histol Histopathol. [Crossref]
- 47. Cho MI, Garant PR (2000) Development and general structure of the periodontium. Periodontol 2000 24: 9-27. [Crossref]
- 48. Yao S, Pan F, Prpic V, Wise GE (2008) Differentiation of stem cells in the dental follicle. J Dent Res 87: 767-771. [Crossref]
- 49. Zhao M, Xiao G, Berry JE, Franceschi RT, Reddi A, et al. (2002) Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res 17: 1441-1451. [Crossref]
- 50. Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, et al. (2007) Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res 329: 283-94. [Crossref]
- 51. Guo W, Gong K, Shi H, Zhu G, He Y, et al. (2012) Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 33: 1291-1302. [Crossref]
- 52. Liu N, Gu B, Liu N, Nie X, Zhang B, et al. (2014) Wnt/β-catenin pathway regulates cementogenic differentiation of adipose tissue-deprived stem cells in dental follicle cell-conditioned medium. PLoS One 9: e93364. [Crossref]
- 53. Yang Y, Ge Y, Chen G, Yan Z, Yu M (2014)Hertwig's epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone 63: 158-65. [Crossref]
- 54. Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H,(2014) Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep 4: 6044. [Crossref]
- 55. Gatten DL, Riedy CA, Hong SK, Johnson JD, Cohenca N (2011) Quality of life of endodontically treated versus implant treated patients: a University-based qualitative research study. J Endod 37: 903-909. [Crossref]
Editorial Information
Editor-in-Chief
Masayoshi Yamaguchi
Emory University School of Medicine
Article Type
Review Article
Publication history
Received: September 15, 2014
Accepted: September 27, 2014
Published: October 01, 2014
Acknowledgements
We thank Drs. Wada, Fujii, Tomokiyo, and Monnouchi for their great supports in preparation of this chapter. This work was financially supported by grants-in-aid (Project Nos. 24390426, 25293388, 25670811, 26462887, 26670825, and 26670826) for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (Japan).
Copyright
Copyright: ©2014 Maeda H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Maeda H, Akamine A (2014). Quest for the development of tooth root/periodontal ligament complex by tissue engineering. Integr Mol Med, 1: DOI: 10.15761/IMM.1000106

