Abstract
With the aim of developing high-performance gas barrier films, the multi-layer coating method that uses ultraviolet irradiation to a polysilazane coating formed on an alicyclic polyimide film has been investigated. The multi-layered gas barrier film consisting of five layers was prepared by using organic polysilazane to form an intermediate stress relaxation layer. This multi-layered film is smooth and uniform, with no cracks, exhibiting superior gas barrier properties. The film is also transparent and highly heat resistant (alicyclic polyimide, Tg: 300°C), making it useful for the formation of flexible devices.
Key words
polysilazane, photo-irradiation, gas barrier characteristics, alicyclic polyimide film, flexible electronics
Introduction
In recent years, significant process has been made in the development of electronic terminal devices such as mobile phones and televisions, and electronics-related products such as solar cells, and there are calls for the development of products with greater functionality and performance [1-4]. In particular, for the next generation of electronic devices, attention is turning to products with flexible properties such as wearable devices, flexible batteries, electronic paper and flexible display devices that are thin, light and flexible [5,6]. A flexible substrate is an essential constituent of such products. Flexible substrates are generally made using flexible organic resin films. However, ordinary organic films do not perform well at blocking the passage of gases such as oxygen, water vapor and the like. This can result in problems such as oxidation of device elements formed on these films, leading to serious performance degradation issues. There is consequently a strong demand for the development of flexible substrates with better gas barrier characteristics. One way of improving the gas barrier characteristics of an organic film is to add a dense inorganic coating, but the low heat resistance of the organic film makes it difficult to form an inorganic gas barrier coating with adequate performance. The most popular approach involves using large-scale high-vacuum film deposition apparatus to form multiple layers of SiO2-based coatings or inorganic oxide coatings by using methods such as sputtering or vacuum deposition to restrict the temperature to which the substrate is heated [7-12]. However, this approach is not only costly due to the use of complex vacuum equipment, but also results in gas barrier characteristics that are still inadequate. Another simpler approach involves coating the organic film with an inorganic precursor solution [13-17], but since it has to be heated to several hundred degrees Celsius in order to form a dense inorganic coating, this approach cannot easily be applied to organic films with low heat resistance.
Recently, organic films with better gas barrier characteristics have been developed by studying methods that use irradiation and polysilazane coatings with excimer light or ultraviolet light to form dense inorganic coatings on PET films at lower temperatures [18-24]. We have successfully developed a highly transparent and highly heat-resistant alicyclic polyimide film and a PET film with good gas barrier characteristics by using ultraviolet rays from a low-pressure mercury lamp to irradiate a polysilazane coating while applying heat [25-26]. In this article, we report on how this method can be used to form multi-layer gas barrier coatings on alicyclic polyimide films with even better performance, and we present the results of examining their characteristics. To explore the causes of this gas barrier performance, we also discuss the results of examining the film’s surface state by AFM, and the film’s electrical properties such as its dielectric breakdown properties.
Experimental
Preparation of silica coatings by using light irradiation
As a precursor for forming thin coatings, a solution of polysilazane (perhydropolysilazane: PHPS) in dibutyl ether (NL-110, 20 wt%: AZ Electronic Materials Co.) was used. This polysilazane solution was diluted to 5 wt% in dibutyl ether and spin-coated onto an alicyclic polyimide film (PI, thickness 100 µm, Mitsubishi Gas Chemical Co.) that had been processed to make it hydrophilic. A low-pressure mercury lamp was used to irradiate the film with light while exposed to the air. The film was irradiated at 12 mW/cm2 for 20 minutes. This irradiation on substrates held at four different temperatures: 80°C, 100°C, 120°C and 150°C was performed. Coatings were applied to both sides of the film. Samples by similar methods at two different spin coating rotation speeds: 800 rpm and 600 rpm were prepared. Comparative samples that were only subjected to heat treatment (at 150°C, 200°C, 250°C and 300°C for 20 minutes) were also prepared.
Preparation of multi-layer coatings
Preparation of methylperhydropolysilazane (MHPS): 100 wt% methylperhydropolysilazane (Toresumairu HTA 1500, Sanwa Kagaku Co.) was diluted with dibutyl ether to form a 5 wt% solution of MHPS.
Preparation of the multi-layer coating: Using a 5 wt% solution of PHPS, two layers of PHPS-derived SiO2 on a PI film according to the method shown in section 2.1 were formed. Light irradiation was performed at an intensity of 12 mW/cm2 for 20 minutes at a substrate temperature of 150°C. This was then covered with a 5% solution of MHPS by spin coating (1000 rpm, 60 seconds), followed by heat treatment at 120°C for 20 minutes to form the intermediate layer (MHPS-derived SiO2) of the multi-layer coating. On top of that, two layers of PHPS-derived SiO2 were formed according to the method using a 5wt% solution of PHPS.
Evaluation
The surface of the coating with an atomic force microscope (AFM:5100N, Hitachi Hightech Science Co.) was observed. The cross-sectional structure of the coating with a transmission electron microscope (TEM:H-9000NAR, Hitachi Ltd.) was also observed. The TEM observation samples were prepared by FIB (Focused Ion Beam) processing. By using X-ray photoelectron spectroscopy (XPS: Quantera II, PHI Co.) an elemental analysis while sputtering from the upper surface of the coating layers was performed. To assess the gas barrier characteristics, the water vapor permeability (40°C, 90% RH: Mocon Permatran-W or Aquatran) was measured. Water vapor transmission rates of 1 g/m2・day or above were measured using the dish method. The water vapor permeability of the multilayer coating was measured at a humidity of 90% RH and at temperatures of 40°C, 50°C and 55°C. The adhesion strength and hardness of the coating on the PI film were evaluated by performing tape peeling and pencil hardness tests according to JIS standards (JIS K5600). The dielectric breakdown field strength was obtained from the voltage–current characteristics of a (single-layer) coating formed on a silicon substrate. An electrode (Au(100 nm)/Ti(10 nm)) on the coating was formed and measured the current flowing through it while varying the voltage between 0 and 20 V. The dielectric breakdown field strength was estimated from the voltage at which there was a sudden increase in current, based on the formula voltage (V)/film thickness (cm).
Results and discussion
Preparation and properties of SiO2 films derived from polysilazane
The coatings formed on polyimide films have very smooth surfaces, and when observed at magnifications of 10,000 to 20,000 with a scanning electron microscope (SEM), it was not possible to observe any differences between coatings formed under different conditions. Therefore AFM measurements were performed, which enable the observation of finer structures.
Figure 1 shows AFM images of (a) the PI surface, (b) the polysilazane (PHPS) coating (before light exposure), and (c) single-layer and (d) two-layer coatings after light exposure at 150°C. In the unexposed coating, it can be seen that the surface state is similar to that of the PI surface. In the exposed single coating surface, it is possible to see small particles of approximately 2–3 nm in size. These are thought to have formed due to the growth of fine SiO2 particles. In the two-layer coating, it can be seen that the surface is even smoother. This is thought to be because the surface irregularities in the single coating surface are smoothed out by being buried under the PHPS solution during the second spin-coating stage.
Figure 2 shows the surface state of coatings after light irradiation and heat treatment (two-layer coatings) while varying the temperature at which the coatings are formed. In the coatings irradiated with light, it can be seen that the occurrence of particles and irregularities decreased with increasing temperature (room temperature→100°C→150°C), resulting in a smoother surface. On the other hand, in the heat-treated coatings, the growth of surface particles increased with increasing heat-treatment temperature (150°C→250°C→300°C) was observed. Light irradiation thus resulted in coating surfaces that were smoother and more compact. The difference in surface states between the light-irradiated coatings and heat-treated coatings suggests that there are different generation and growth mechanisms at play in the transition from PHPS to an SiO2 coating, although the details of these mechanisms are currently not clear.
Figure 3 compares the surface hardness (pencil hardness test) values of the light-exposed and heat-treated coatings. Since a dense SiO2 coating has greater surface hardness, it can be used as one indicator of how dense the coating is. As the polysilazane coating heat treatment temperature was increased from 150°C to 300°C, the surface hardness increased from 3H to 6H. On the other hand, the surface hardness of the light irradiated film also increased with as the temperature at the time of light irradiation increased — from 4H at room temperature to 9H at 120°C or above. The increased surface hardness is due to resulting from increased conversion of the polysilazane coating to silica (SiO2), and increasing compactness of this coating. It is thought that the increased silica (SiO2) conversion and the increased compactness of this coating with light irradiation occurred at temperatures substantially lower than those used for heat treatment. Changes in the coating’s chemical structure during light irradiation can be tracked by examining the infrared absorption spectrum. It has already been reported that the transition from polysilazane coating to silica takes place at temperatures much lower than those required for heat treatment [25,26]. In the oxidation reaction of the polysilazane coating, it is inferred that contributions are made by the oxidation effects of O3 and O(1D) active oxygen derived from atmospheric oxygen, and by the chemical bond cleavage effects of optical energy supplied by the low-pressure mercury lamp at wavelengths of 254 nm and 185 nm. The energy levels of light with wavelengths of 254 nm and 185 nm are 113 and 155 kcal/mol, respectively. The Si-N and N-H bond energies of polysilazane are 105 and 92 kcal/mol, respectively, while the low-pressure mercury lamp produces light with more energy than these bonds, allowing it to break these bonds efficiently. An Si atom whose bonds have broken immediately reacts with the activated oxygen to form SiO2. The substrate temperature increases the rate of activated oxygen diffusion and promotes oxidation reactions in the vicinity of polysilazane bonds that have been broken by the optical energy, which contributes to the formation of inorganic SiO2.
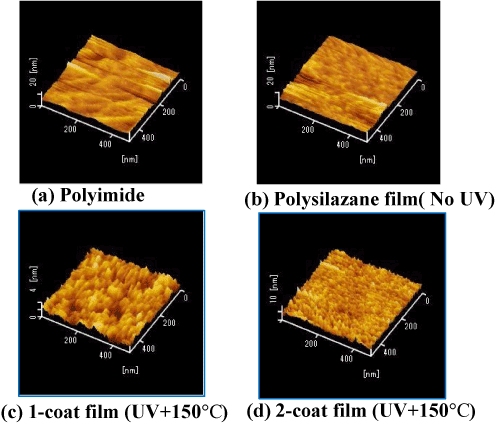
Figure 1. Surface AFM images of polysilazane-derived SiO2 films
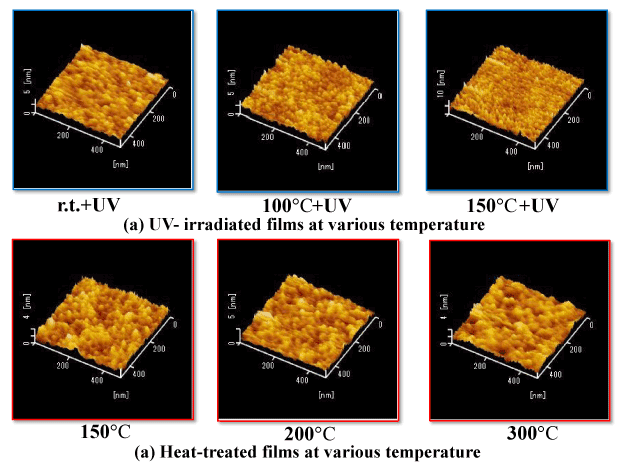
Figure 2. Comparison of surface AFM images of UV-irradiated and heat-treated films
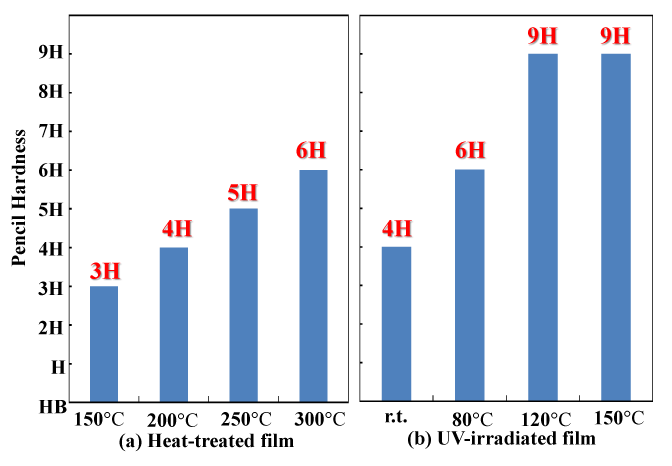
Figure 3. Surface hardness of heat-treated and UV-irradiated films
Figure 4 shows the current–voltage characteristics of the polysilazane film. From the voltage at which the current increases sharply, the dielectric breakdown field strength of the oxide coating can be estimated, which is one indicator of the insulating film’s compactness. This polysilazane film substrate was compared with single-coating films formed on a Si substrate. The figure also shows the results for two coating layers formed by light irradiation at 150°C. For the light irradiated films at temperatures ranging from room temperature up to 120°C, the current increased suddenly at around 10–11 V (the region above 10 V is not shown for this reason). For the light irradiated at 150°C, the sudden current jump occurred at 15.6 V. Based on this value, the dielectric breakdown strength of the coating is 1.2 MV/cm. Meanwhile, the two-layer coating irradiated with light at 150°C underwent no large change in DC current even when subjected to 20 V, showing that it had a large dielectric breakdown field strength. This demonstrates that the use of multiple layers makes the coating denser. On the other hand, the DC current increased rapidly at 5.5 V in the coating subjected to heat treatment at 150°C. Coatings subjected to light irradiation are denser than coatings subjected to thermal processing. The compactness of a coating is thought to have a major effect on the improvement of gas barrier characteristics.
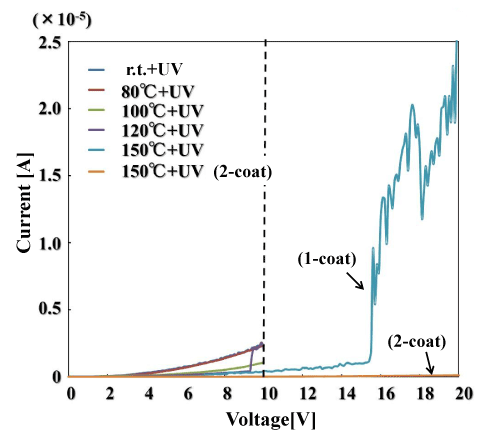
Figure 4. Breakdown field strength of UV-irradiated films
Figure 5 shows the gas barrier characteristics of the polysilazane films. The water vapor transmission rate of the heat-treated coating decreased from 143 g/m2 ・ day for the ordinary PI film, to 48.6 g/m2 ・ day for coatings heat treated to 150°C, 36.9 g/m2 ・ day for 200°C, 2.26 g/m2 ・ day for 250°C, and 1.14 g/m2 ・ day for 300°C. On the other hand, for the coatings subjected to light irradiation, we obtained better gas barrier characteristics than with heat-treated coatings when heating to 80°C (1.77 g/m2 ・ day)→100°C (1.22 g/m2 ・ day)→150°C (0.08 g/m2 ・ day). This demonstrates that our method is effective for the formation of gas barrier coatings at low temperatures.
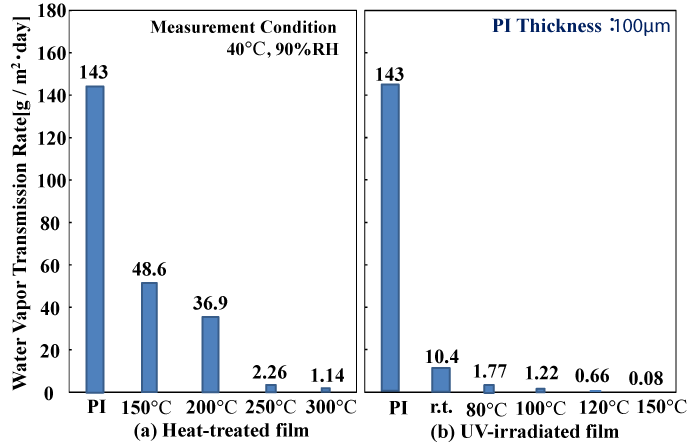
Figure 5. Water Vapor Transmission Rate of polysilazane-derived SiO2 films (2-coat)
Figure 6 shows the gas barrier characteristics of samples obtained while varying the spin coating rotation speed used to form the polysilazane coatings. As the rotation speed decreases from 1000 rpm to 800 rpm and 600 rpm, the transmission rate decreases from 0.08 g/m2 ・ day at 1000 rpm to below 0.02 g/m2 ・ day, which is lower than the measurement limit of the Mocon Permatran apparatus used to perform these measurements. It is thought that a lower rotation speed leads to an increase in the film thickness, and thus has a large effect on the gas barrier characteristics of the polysilazane coating.
Preparation and properties of multi-layer coatings
Increasing the number of gas barrier layers is highly effective at improving the gas barrier characteristics. However, when four or more layers of polysilazane coatings are simply applied one on top of the other, cracks are liable to form, resulting in inferior characteristics. This is thought to be because the addition of more layers makes the properties of the inorganic SiO2 glass more prominent, increasing the likelihood of stresses due to the difference in thermal expansion coefficients between the glass coating and the organic PI film substrate. We therefore decided to form a laminated coating with an intermediate layer of organic polysilazane (MHPS) (Figure 7b). As shown in Figure 7a, organic polysilazane (MHPS) has a chemical structure that consists of CH3 groups connected to a polysilazane backbone. When heated in air, it transforms into SiO2 with CH3 groups. Since this coating has organic CH3 groups in an SiO2 backbone, it adds flexibility to the coating. Also, since it is sandwiched between layers of polysilazane above and below, which have a similar molecular structure, this structure has good consistency between the multiple layers. Therefore, it is thought that a laminated gas barrier coating is suitable as a stress-relieving layer. Figure 8 shows optical micrograph images of the surface state of a four-layer (PHPS-SiO2) structure and a five-layer (PHPS-SiO2 (2-layer)/MHPS-SiO2 (1-layer)/PHPS-SiO2 (2-layer)) structure incorporating a stress-relieving layer (stress relaxation layer). Although cracks have formed in the surface of the four-layer structure, the stress-relieving five-layer structure has a smooth surface with no cracks.
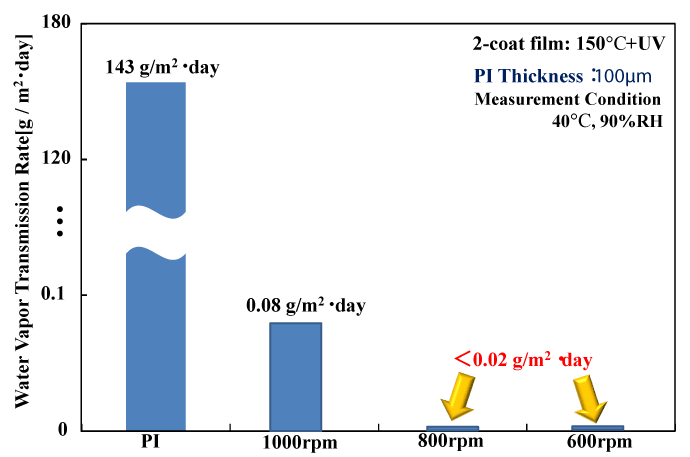
Figure 6. Film thickness dependency of WVTR for UV-irradiated films at 150℃
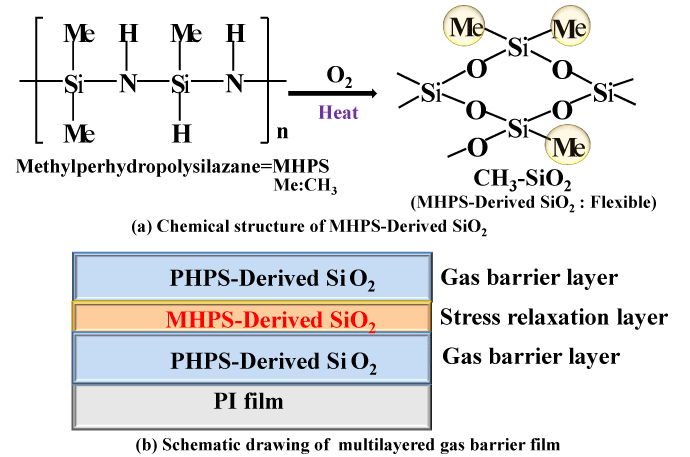
Figure 7. Chemical structure of MHPS-Derived SiO2 and schematic drawing of multilayered gas barrier film
Figure 9 shows a cross-sectional TEM image of the multi-layer coating film that consists of two coats of polysilazane formed by spin coating at 800 rpm (220 nm thick), one coat of organic polysilazane (35 nm thick), and a further two coats of polysilazane (240 nm thick). In the two coats of polysilazane at the top and bottom of this structure, any interface between the polysilazane layers (PHPS derived SiO2) were not observed, and the polysilazane layers have a uniform appearance. There are also uniform interfaces between the polysilazane (PHPS derived SiO2)/organic polysilazane (MHPS derived SiO2) layers and the polysilazane (PHPS derived SiO2)/PI film layers. No cracks formed in the multi-layer coating due to the formation of organic polysilazane as an intermediate stress-relieving layer, and tape pealing tests showed that the coating was strongly bonded with an adhesion strength of 100/100 (remaining number of sheets/ number of cuts).
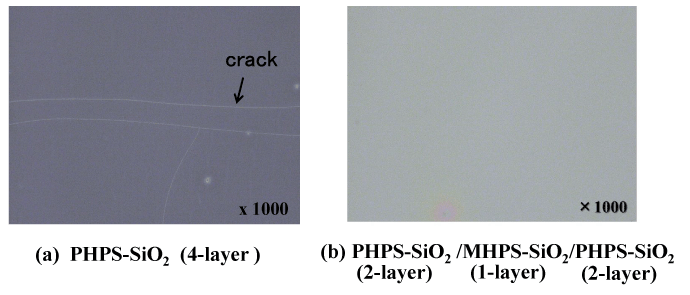
Figure 8. Optical microscope photographs of surface of the multilayered gas barrier films
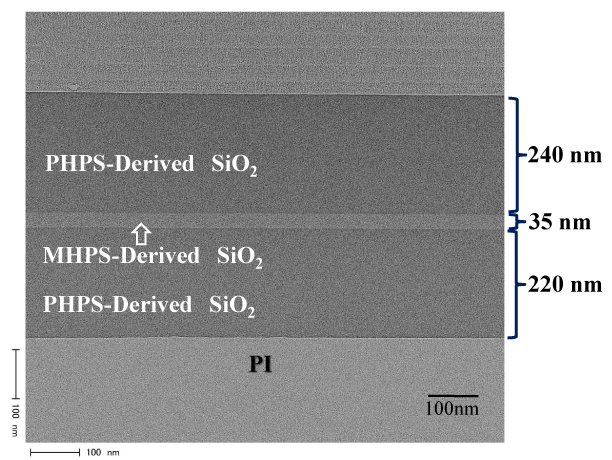
Figure 9. Cross sectional TEM photograph of the multilayered gas barrier films
Figure 10 shows the results of elemental analysis in the depth direction by XPS. Four elements: silicon (Si), oxygen (O), nitrogen (N), and carbon (C) were measured. The small amount of C present on the sample surface is thought to originate from surface contamination with organic material. The presence of Si, O, C and N in the multi-layer coating (except at the vicinity of the polyimide interface) was observed. During sputtering times of approximately 3–56 minutes and 64–116 minutes, the abundance ratio of Si is approximately 32 at% and the abundance ratio of O is approximately 67 at%. These Si and O atoms are thought to originate from SiO2 formed by the polysilazane (PHPS). The composition of the SiO2 originating from the polysilazane in the layers above and below the middle layer is very similar. The Si:O composition ratio is thought to be roughly 1:2. In this region, any N or C were hardly observed. Next, in the intermediate layer over the sputtering range from 56–64 minutes, the abundance of Si is slightly reduced and the abundance of O is greatly reduced. At roughly 60 minutes, the composition was approximately 30 at% Si and 49 at% O. On the other hand, the abundance of C and N increased in this range, reaching approximately 17 at% C and 4 at% N at around 60 minutes. The C in this range is thought to originate from the CH3 groups of organic polysilazane (MHPS). N was also found to be present, but is thought to have originated from unreacted sites in the organic polysilazane. It is presumed that chemical species such as Si- CH3,SiOx (x<2) and SiON are present in this intermediate layer. When processing the organic polysilazane coating, only heat treatment at 120°C was performed so as to avoid decomposition of Si-CH3 by light energy. It is thought that this low-temperature processing resulted in some of the unreacted organic polysilazane being left behind.
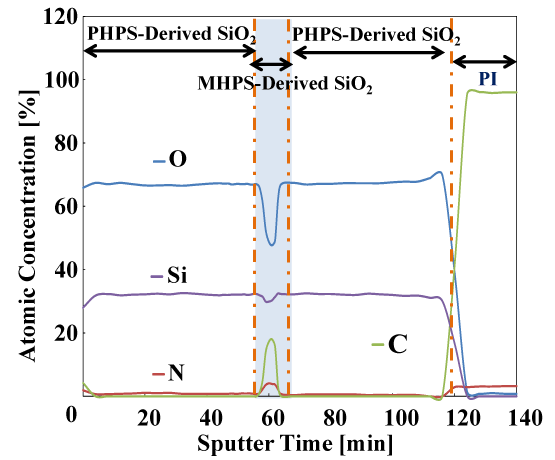
Figure 10. XPS depth profiles of elemental analysis of multilayered gas barrier films
The temperature dependence of water vapor permeability in this multi-layer coating was measured. Figure 11 shows the results of plotting the natural logarithm of water vapor permeability (measured at 90% RH) against the reciprocal of the temperature (1/T) for temperatures of 40°C, 50°C and 55°C. The three measured points (40°C: 0.013 g/m2 ・ day, 45°C: 0.73 g/m2 ・ day, 50°C: 2.15 g/m2 ・ day) have a linear relationship. The activation energy of water vapor transmission (obtained from the slope of the straight line by using the Arrhenius equation) is 436 kJ/mol. The water vapor permeability at room temperature (25°C), which is calculated from the extrapolated straight line, is the very low value of 3.18×10−6 g/m2 ・day. The same linear dependence of water vapor permeability was also observed in gas barrier films formed on PET film [26]. In this case, the room-temperature water vapor permeability and activation energy were 4.50×10−4 g/m2 ・ day and 219 kJ/mol, respectively. Compared with this value, the multi-layer gas barrier film proposed in this study has superior gas barrier characteristics.
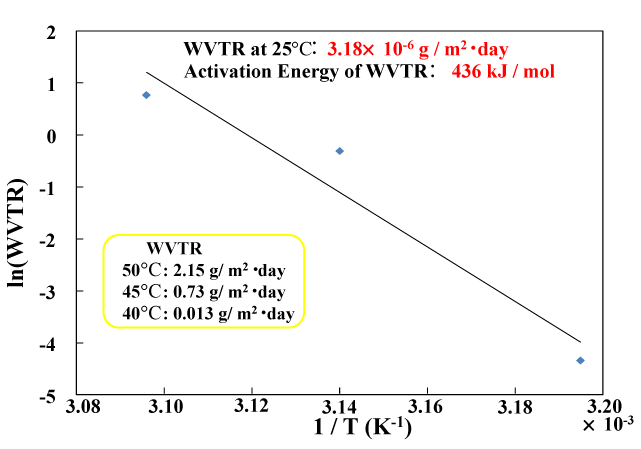
Figure 11. Temperature dependency of WVTR of the multilayered gas barrier films
The formation of an intermediate layer in order to relieve stress in a multi-layer polysilazane coating has already been reported in connection with the use of organic polymers such as polyvinyl alcohol (PVA) [27-28], but was considered to be problematic when interfacing between inorganic and organic materials or the like. The organic polysilazane (MHPS) in this study has a molecular structure that is similar to polysilazane (PHPS) and is able to bond with the polysilazane-derived SiO2 layers above and below the intermediate layer. It thus has a strong adhesion force and is believed to lead to improved reliability of the gas barrier film. The multi-layer film is transparent, and the heat resistance of the alicyclic polyimide film (Tg: 300°C) is also high. It is therefore likely to be useful as a flexible substrate for devices such as flexible displays.
Conclusion
With the aim of developing high-performance gas barrier films, the multi-layer coating method that uses ultraviolet irradiation to a polysilazane coating formed on an alicyclic polyimide film has been investigated. By using organic polysilazane to form an intermediate stress relaxation layer, the gas barrier coating consisting of five layers was prepared. This multi-layer coating is smooth and uniform, with no cracks. Films on which this multi-layer coating has been applied exhibit superior gas barrier properties. The temperature dependence of the film’s water vapor permeability indicates a linear relationship, from which it is inferred that the room temperature gas permeability is just 3.18×10−6 g/m2 ・ day. The film is also transparent and highly heat resistant, making it useful for the formation of flexible devices.
Acknowledgement
This work was supported by JSPS KAKENHI Grant Number 26420711.
References
- White MS, Kaitenbrunner MG, Istrokowacki ED, Gutnichenko K, Kettlgruber G, et al. (2013) Ultrathin highly flexible and stretchable PLEDs. Nature Photonics 7: 811-816
- Sekitani T, Someya T (2011) Human-friendly organic integrated circuit. Materials Today 14: 398-407
- Sekitani T1, Zschieschang U, Klauk H, Someya T (2010) Flexible organic transistors and circuits with extreme bending stability. Nat Mater 9: 1015-1022. [crossref]
- Nomura K, Ohta H, Kamiya T, Hirano M, Hosono H (2004) Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 432: 488-492
- Edited by Wong WS, Salleo A (2009) Flexible Electronics: Materials and Applications, Springer, New York.
- Sekitani T, Someya T (2010) Stretchable, large-area organic electronics. Adv Mater 22: 2228-2246. [crossref]
- Carcia PF, Mclean RS, Groner MD, Dameron AA, George SM (2009) Gas diffusion ultrabarriers on polymer substrates using Al2O3 atomic layer deposition and SiN plasma-enhanced chemical vapor deposition. Journal of Applied Physics 106: 023533
- Yagi Y, Akashi K, (2021 Copyright OAT. All rights reservlm substrates designed for organic electroluminescence device. Journal of Vacuum Society of Japan 50: 735
- Hanada T, Negishi T, Shiroishi I, Shiro T, (2010) Plastic substrate with gas barrierlayer and transparent conductive oxide thin film for flexible displays. Thin Solid Films 518: 3089
- Hata T, Nakayama H, (2008) An organic catalytic CVD: Principle, apparatus and applications. Thin Solid Films 516: 558
- Carcia PF, MacLean RS, Reilly MH, Groner MD, George SM (2006) Catalyst of Al2O3 gas diffusion barriers grown by atomic layer deposition on polymer. Applied Physics Letters 89: 031915
- Dameron AA, Davidson SD, Burton BB, Carcia PF, MacLean RS, et al. (2008) Gas diffusion barriers on polymer using multi layers fabricated by Al2O3 and rapid SiO2 atomic layer deposition. Journal of Physical Chemistry C 112: 4573-4580
- Brinker CJ, Schere GW (1990) Sol-Gel Science. Academic Press, Boston
- Seyferth D, Wiseman GH (1984) High-yield synthesis of Si3N4/SiC ceramic materials by pyrolysis of a novel polyorganosilazane. Journal of American Ceramic Society 37: 132
- Kamiya K, Oka AI, Nasu H, Hashimoto T (2000) Comparative study of structure of silica gels from different sources. Journal of Sol-Gel Science & Technology 495-499
- Iwamoto Y, Sato K, KatoT, InadaT, Kubo Y (2005) A hydrogen-perselective amorphous silica membrane derived from polysilazane. Journal of European Ceramic Society 25: 257
- Funayama O, TshiroY, Kamo A, Okumura M, Isoda T (1994) Conversion mechanism of perhydropolysilazane into silicon nitride-base ceramics. Journal of Material Science 29: 883-4888
- Ohishi T (2003) Gas barrier characteristics of a polysilazane film formed on an ITO-coated PET substrate, J Non-Cryst Solids 330: 248
- Naganuma Y, Tanaka S, Kato C, ShindoT (2004) Formation of silica coating from perhydropolysilazane using vacuum ultraviolet excimer lamp. J Cera Soc Japan 112: 599
- Prager L, Dierdorf A, Liebe H, Naumov S, Stojanović S, et al. (2007) Conversion of perhydropolysilazane into a SiOx network triggered by vacuum ultraviolet irradiation: access to flexible, transparent barrier coatings. Chemistry 13: 8522-8529. [crossref]
- Prager L1, Dierdorf A, Liebe H, Naumov S, Stojanović S, et al. (2007) Conversion of perhydropolysilazane into a SiOx network triggered by vacuum ultraviolet irradiation: access to flexible, transparent barrier coatings. Chemistry 13: 8522-8529. [crossref]
- Kobayashi Y, Yokota H, FuchitaY, Takahashi A, Sugawara Y (2013) Characterization of gas barrier silica coating prepared from perhydropolysilazane films by vacuum ultraviolet irradiation. J Ceram Soc Japan 121: 215
- Morlier A, Cros S, Garandet J-P, Alberola N, (2012) Thin gas-barrier silica layers from perhydropolisilazane obtained through low temperature curing: A comparative study. Thin Solid Films 524: 62-66
- Naganuma Y, Horiuchi T, Kato C, Tanaka S (2013) Low-temperature synthesis of silica coating on a poly(ethylene terephthalate) film from perhydropolysilazane using vacuum ultraviolet light irradiation. Surface & Coatings Technology 225: 40-46
- [Morlier A, Cros S, Garandet J-P, Alberola N (2014) Structural properties of ultraviolet cured polysilazane gas barrier layers on polymer substrates. Thin Solid Films 550: 85-89
- Ohishi T, Sone S, Yanagida K (2014) Preparation and gas barrier characteristics of polysilazane-derived silica thin films using ultraviolet irradiation. Materials Sciences and Applications 5: 105-111
- Ohishi T, Yamazaki Y, Nabatame T (2016) Preparation, structure and gas barrier characteristics of polysilazane-derived silica thin films formed on PET by simultaneously applying ultraviolet-irradiation and heat-treatment. Frontiers in Nanoscience and Nanotechnology 2:149-154
- Morlier A, Cros S, Garandet J-P, Alberola N (2013) Gas barrier properties of solution processed composite multilayer structures for organic solar cells encapsulation 115: 93-99
- Blankenburg L, Schrodner M (2015) Perhydropolysilazane derived silica for flexible transparent barrier foils using a reel-to-reel wet coating technique: Single- and multilayer structures. Surface & Coatings Technology 275: 193-206











