Abstract
Aim: Hypoxic fetal compromises are cured by the early delivery with C-section at present, while the surgery causes maternal morbidity by uterine scar. The vaginal delivery of normal fetus after treatment in utero will cure the fetus reducing placenta accreta in succeeding delivery.
Methods: 1. Maternal O2 inhalation increased fetal PaO2 in fetal distress, though the effect was insufficient. 2. Placental Intervillous fibrin deposit was solved by heparin improving fetal growth restriction and fetal hypoxia. 3. Improved umbilical cord circulation by maternal posture change, 4. Pharmaceutical anti-glutamate and free radical scavenger etc. were expected. 5. TRAP sequence and fetal anomaly was treated by high-intensity focused ultrasound. 6. Improved uterine maternal circulation by tocolysis in hypercontraction; uterine arterial constriction in preeclampsia will be dilated by antisympathicotonic therapy; 7. Future stem cell therapy of tissue defects and neurological damage, genetic diseases treated by chromosomal engineering and gene editing.
Results and conclusion: The success of No. 1-3 topics have been reported, while No. 5-7 will be achieved in the future. Preterm labor is particular problem in CP reduction.
Key words
fetal hypoxia, brain damage, cerebral palsy, C-section, FHR, placenta accrete, fibrin deposit, high-intensity ultrasound, stem cell, gene therapy
Introduction
Fetal arterial blood oxygen pressure (PaO2) is about half of adult, and umbilical arterial PaO2 was lower than 50 mmHg [1] therefore fetus tends to be hypoxic, due to the oxygen supply from circulating maternal arterial blood in the placenta. Fetal hypoxia damaged central neuronal cells [2]. Placenta villi prepares active transfer function of nutritive materials for fetal growth, e.g. glucose concentration of fetal blood is higher than maternal blood, while oxygen and carbon dioxide gasses are transferred by passive function. Therefore, the active transfer function is damaged forming fetal growth restriction (FGR), preserving simple passive gas transfer, therefore fetal hypoxia appears in FGR after the fetal weight reduction, i.e. FHR acceleration disappears against fetal movement in early stage of hypoxia, where FHR variability is preserved, which is provoked by minor fetal movements. Then suddenly appear severe hypoxic FHR, composed of the loss of variability, severe late decelerations and bradycardia within 2 weeks after the loss of acceleration, where all cases received C-section, but the outcome was ominous, developing severe neonatal asphyxia or death, while FGR cases of normal acceleration was born in normal vaginal delivery [3]. Therefore, fetal brain damage due to hypoxia should be prevented in FGR cases.
Methods
Maternal oxygen inhalation
O2 gas inhalation for 2 L per min was usually performed in cases of fetal distress, where umbilical arterial PO2 elevated, but ineffective to disappear fetal hypoxia in the past [1].
Treatment of fibrin deposit in placental intervillous space
Utsu & Maeda [4] found high echogenesity and high GLHW tissue characterization value in a FGR case in the second trimester, detecting fibrin deposit (Figure 1) in intervillous space by ultrasound, treated with 5,000 U heparin every day, where fetal weight increased to normal level, no hypoxic sign was found, and normal neonate was obtained, instead of fetal death due to FGR in previous pregnancy. Therefore, a FGR case would be treated with fibrin solution therapy, if fibrin deposit is diagnosed by high ultrasound image echogenesity and GLHW tissue characterization. The fibrin deposit would be treated even in the loss of FHR acceleration, to prevent severe hypoxia in FGR, where the recovery of acceleration should be confirmed. The FHR in the loss of FHR variability is such severe fetal brain damage as the anencephalic FHR, so that C-section should be performed before the loss of variability [5]
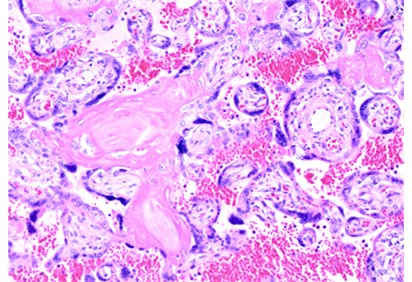
Figure 1. Microscopic fibrin deposit in intravillous space of a FGR placenta.
H-E, 200X (Utsu M)
Improved umbilical cord circulation in variable deceleration by maternal posture change.
Pharmaceutical treatment
The application of antiglutamate [6], free radical scavenger and others have been proposed.
Intensive focused ultrasound ablation
The ablation of nutritive vessels to acardiac twin (TRAP sequence) was achieved by the non-invasive high-intensity focused ultrasound [7]. Various fetal abnormalities will be noninvasively treated by the technique, where associated hypoxia will be treated.
Treatment of hypoxia due to uterine artery constriction in preeclampsia
The constriction of uterine artery, radial and spiral arteries reduce maternal arterial blood flow in the placenta resulting placental infarction, fetal hypoxia and fetal death (Figure 2) due to the sympathicotony in preelampsia [8]. Therefore, anti-sympaticotonic treatment will prevent the hypoxia in the future.
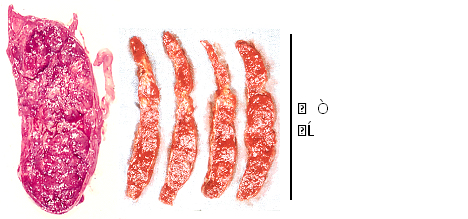
Figure 2. Placental infarction in a preeclampsis complicated by fetal death.
(Maeda 1965)
Congenital diseases develop fetal hypoxia, where stem cell therapy will treat tissue defect and neurological damage, and genetic diseases will be treated by chromosomal engineering and gene editing in the future, where associated hypoxia will be removed.
Results
Fetal body weight increased to normal level, no hypoxic sign was recorded, and normal newborn was obtained after fibrin deposit solution therapy with heparin administration to the mother in a case of FGR, which associated placental fibrin deposit, while the fetus without fibrin deposit solution treatment died in previous pregnancy of the same mother [4].
Since preeclampsia is caused by the excitation of hypothalamic sympathetic center [8] uterine artery and its branch arteries would be constricted inhibiting placental maternal blood flow to cause placental infarction and fetal hypoxia causing fetal death in the past. Therefore, anti-sympathicotonic treatment will treat pregnancy induced hypertension, organ damages and fetal hypoxia in the future.
Maternal posture change was effective to improve variable deceleration reducing umbilical cord compression. New local labor anesthesia in the vagina [9] reduced pelvic floor resistance increasing vaginal delivery.
Discussion
Cesarean section rate performed by obstetric indication was about 8 % and by fetal hypoxic NRFS was 3% of all births, namely actual C-section number in one million births in Japan will be about 30,000 cases in a year preventing fetal damage due to hypoxia. The effort to reduce C-section under the diagnosis of NRFS (fetal hypoxia) will be useful to lower the maternal morbidity.
Since fetal hypoxia is primarily caused by decreased placental villi oxygen transfer from maternal blood, increased maternal arterial blood flow removing inter-villous fibrin deposit will treat fetal hypoxia. Also, the increase of maternal blood flow in the placenta removing sympathicotonic constriction of uterine artery and its branches will improve fetal hypoxia in preeclampsia. Maternal hypoxia or hypotension caused by maternal disorders or massive hemorrhage can cause fetal hypoxia, which will be improved by maternal treatment.
Secondly, various fetal disorders including congenital diseases complicated by fetal cardiac failure will develop fetal hypotension reducing fetal placental circulation and oxygen intake. Therefore, the treatment of fetal disorders will improve fetal hypoxia in the case. Noninvasive high-intensity focused ultrasound ablated nourishing vessel of a TRAP sequence would improve the donor twin [7]. Various fetaj diseases will be treated using the technique improving fetal disorders and hypoxia in the future. Such genetic disorders as trisomies would be treated by chromosomal technology [10] and gene editing [11] improving fetal hypoxia in fetal disorders in the future.
In preterm labor, periventricular echodensity (PVE), which is hyperechogenesity around the ventricle, preceded neonatal PVL and CP, when the PVE lasted until preterm birth, but no brain damage was noted in the full term delivery [12]. Therefore, normal infant is expected without hypoxia when preterm labor is suppressed by tocolytic therapy until full term delivery.
Conclusion
Although the treatment of fetal hypoxia usually depends on C-section at present, obstetricians would endeavor to find such non-surgical therapy as the solution of fibrin deposit in placental intervillous space, or antisympathetic therapy in preeclampsia. Various maternal, fetal or genetic diseases will be treated in the future to achieve normal vaginal delivery. Preterm labor will be treated to prevent CP.
2021 Copyright OAT. All rights reserv
References
- Maeda K, Kimura S, Nakano H, Fukui Y, Ozawa S, et al. (1969) Pathophysiology of Fetus. Fukuoka Printing, Proc, 21st JSOG Conv, Shukudai lecture, 1969.
- Windle WF (1966) An experimental approach to prevention or reduction of the brain damage of birth asphyxia. Develop Med Child Neurol 8: 129-140. [Crossref]
- Teshima N (1993) [Non-reactive pattern diagnosed by ultrasonic Doppler fetal actocardiogram and outcome of the fetuses with non-reactive pattern]. Nihon Sanka Fujinka Gakkai Zasshi 45: 423-430. [Crossref]
- Maeda K, Utsu M, Kihaile PE (1998) Quantification of sonographic echogenicity with grey-level histogram width: a clinical tissue characterization. Ultrasound Med Biol 24: 225-234. [Crossref]
- Maeda K (2014) Origin of the long-term variability and acceleration of FHR studied for the prevention of cerebral palsy in fetal hypoxia and general insults. J Perinat Med 42: 401-403. [Crossref]
- Kochhar A, Justin A, Patrick D, Mazzarella V (1988) Glutamate antagonist therapy reduces neurologic deficits produced by focal central nervous system ischemia. Arch Neurol 45: 148. [Crossref]
- Okai T, Ichizuka K, Hasegawa J, Matsuoka R, Nakamura M, et al. (2013) First successful case of non-invasive in-utero treatment of twin reversed arterial perfusion sequence by high-intensity focused ultrasound. Ultrasound Obstet Gynecol 42: 112-114. [Crossref]
- Maeda K (2014) Preeclampsia is caused by continuous sympathetic center excitation due to an enlarged pregnant uterus. J Perinat Med 42: 233-237. [Crossref]
- Utsu M, Kato Y, Takehara K, Maeda K (2016) Safe labor analgesia with vaginal submucosal injection and pudendal nerve block. Global J Anestheology 3: 011-013.
- Oshimura M, Uno N, Kazuki Y, Katoh M, Inoue T (2015) A pathway from chromo- some transfer to engineering resulting in human and mouse artificial chromosomes for a variety of appliations to biomedical challenges. Chromosome Res 23: 111-33. [Crossref]
- Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, et al. (2016) Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci Rep 6: 22555. [Crossref]
- Yamamoto N, Utsu M, Serizawa M, Ohki S, Murakoshi T, et al. (2000) Neonatal periventricular leukomalacia preceded by fetal periventricular echodensity. Fetal Diagn Ther 15: 198-208. [Crossref]


