Abstract
In Entamoeba stem cells, oxygen availability regulates proliferation and differentiation. In the past Entamoebae were categorized as “micro-aerophile” organisms. Later, they were considered to be anaerobes because they perform an anaerobic/ fermentative metabolism and possess mitosomes/ dehydrogenosomes instead of mitochondria. Since the discovery of the stem cell lineage of Entamoeba it is known that the pathogen amoebae have a primary multipotent stem cell line (p-SRL) that converted in two progenitor stem cell lines of reduce potency producing a single type of differentiate cell (MAS or MAT) each. Both progenitor lines preferentially colonize the cecum that provides usually an oxygen gradient of <1%-5.5% O2 content. The s-SRL line is an oxygenic cell line of limited self-renewal residing in the upper oxygenic zones; it proliferates by fast cycling and differentiates to ATD cysts by intrinsic mechanisms of terminal differentiation via committed MAS cells. MAS cells are precursor cells for natural encystment and form ATD cysts. The other cell line is the unipotent t-SRL line living in all gradient zones. It switches from fast to slow cycling and back again and differentiate non-committed MAT cells. Hypoxia <1% O2 amplifies the t-SRL line by complete self renewal (symmetric cell fate) preserving the undifferentiated hypoxic cell state (ISH cells). Declining hypoxia reconstitutes the slow cycling t-SRL line. Oxygen depletion represses terminal differentiation: first, it forces the s-SRL line to converse into a non-encysting t-SRL line and secondly, it interrupts precursor cell progression through the developmental cell cycle.
Key words
Entamoeba invadens, endopolyploid cell cycle, committed mature and immature precursor cells, check point for terminal differentiation, symmetric cell fate, undifferentiated hypoxic cell state
Abbreviations
p-SRL, s-SRL, t-SRL: self renewing primary, secondary and tertiary cell lines; SRP cells: self renewing primary stem (P) cells; SRS, SRT: self renewing secondary (-S) and tertiary (-T) cells/D1 cells; P/S, P/T, S/T: cell line conversions; MAP, MAS, MAT cells: mitotic arrested primary, secondary and tertiary cells/D2 cells; RSC: reserve stem cells (primary cells); ISH, ILH: identical strong and low hypoxic cells; ATD, ITD: autonomous and induced terminal differentiation (encystment); TD check point: checkpoint for terminal differentiation; OCB: oxygen consuming bacteria; AaEM: encystment medium; EMHU: encystment medium containing hydroxyurea; HIF: hypoxia inducible factor.
Introduction
In their natural intestinal habitat, the stem cell lines of the pathogen Entamoeba species E. invadens and E. histolytica move constantly between more oxygenic to more hypoxic areas[1] containing between 0.15% and ~5.5% O2. This is the oxygenic scale that defined the intestinal residence of amoebic stem cells. In fact, amoebic populations are exposed first to the intraluminal steep oxygen gradient and secondly to the oxygen gradient that exists between the hypoxic lumen and the perivascular areas of mucosa, submucosa and serosa [1,2]. The intestinal lumen is essentially anoxic. Previous studies on parasitic amoebae only paid attention to the balance of nutrients, bacterial associates and host immune responses and give very little attention to the oxygen concentration both in vivo and in cultures. First, the OCB culture method developed for E. invadens introduced ascending and descending oxygen gradients in amoebic cultures. The reward of this innovation was the discovery of the stem cell protolineage of Entamoeba.
Stemness is an old eukaryotic aquisition and all basic characteristics of stemness are conserved both in protists such as Entamoeba and higher organisms such as mammals. Entamoeba colonize preferentially the cecum of the host and the intestinal wall where oxygen concentrations vary between <1% and ~6% O2. It is generally accepted that, in stem cells, oxygen and hypoxia control regulation of cell fate and cell differentiation: while oxygenation is decisive for differentiation, hypoxia promotes an undifferentiated cell state in several stem cell and precursor cell populations [2]. This is a hallmark of all stem cell lineages. In addition, it is the idea that single celled eukaryotes conserving the ancestral mechanisms of stemness had more opportunities for the parasitic/invasive way of life [3].
Oxygen gradients modulate cell proliferation and quiescence and control molecular mechanisms such as translation initiation of mRNA by protein regulators of the eukaryotic initiation factors 4E (eIF4E) [4]. Three pO2 levels are physioxicly significant regarding the stem cell system:
- Oxygen tension of 5% -7% that favor the differentiation process
- Oxygen tension of 3%-5% have no effect on proliferation [5] however it reduce significantly differentiation;
- ≤1% oxygen availability decrease or stop proliferation
O2 measurements in the cecum revealed baseline pO2 levels of about 5.5% O2 (40 mmHg) and luminal pO2 levels below 0.14% O2 (1 mmHg) [6]. According to the authors, the oxygen gradient extends initially from the tissue throughout the mucosal interface into the lumen however microorganisms adjacent to the tissue consume most of the available oxygen, keeping the intestinal lumen anaerobic. Measurements suggest that the oxygen-containing layer - which is the closest to the tissue - is very thin and the oxygen gradient in the lumen must be very steep. The dynamics of oxygen consumption at the mucosal interface by bacteria modulate Entamoeba development.
Our knowledge of Entamoeba’s multipotent p-SRL stem cell line is limited [7]. Primary cells of E. histolytica come into appearence in the small intestine - possibly in duodenum - where the metacyst hatched out and the amoebulae generate to the primary p-SRL line. It is a short living stem cell line that proliferates asymmetrically at the lumen-mucus interface in the small intestine. The p-SRL line consists of self-renewing SRP cells that proliferate and divide by asymmetric cell division producing a new SRP cell and a mitoticly quiescent MAP cell. MAP cells reside as reserve stem cells in adequate RSC niches (zones) of the small intestine wall. The self-renewal of the p-SRL line is limited to the primary culture formed by hatching cysts; by passaging in subculture, the p-SRL line converses to one of the two progenitor cell lines s-SRL or t-SRL that give rise to more differentiated progeny. Both progenitor cell lines are of reduced potency.
The oxygenation of the exhausted (pre-) stationary OCB culture sediments by stirring and aeration converts the oxygenicly stressed p-SRL to a secondary s-SRL line by P/S conversion. The secondary s-SRL is an absolute oxygenic cell line. It proliferates by fast cycling (cell cycle duration: 6 hrs) producing MAS cells as committed precursor cells for autonomous terminal differentiation (ATD encystment). In other words, high oxygen conditions facilitate differentiation increasing SRS cell proliferation. Although Entamoeba cells have an anaerobic fermentative metabolism, oxygen promotes mitotic progression and terminal differentiation.
Advanced oxygen depletion stops s-SRL proliferation and converse it to a tertiary t-SRL line. The t-SRL line is a facultative hypoxic progenitor cell line of unlimited self renewal (long term cell line, LT line). It uses the entire hypoxic/ physioxic spectrum of the intestine for vegetative life and does not produce ATD cysts. However, it can be induced to form ITD in nutrient free media. In high oxygenic environments the t-SRL proliferates by fast cycling (6 hrs lasting cell cycle) and in more hypoxic environments by slow cycling. High O2 condition improves proliferation efficiency of the hypoxia resistant t-SRL line [7].
Concerning its provenance, the t-SRL line has different origins: one of them is the S/T variant originated from the s-SRL line and the other is the P/T variant originating directly from the p-SRL; another variant is the ISH/T variant formed by hypoxic amplified ISH cells [2,7]. The cycling t-SRL line produces mitotic quiescent uncommitted cells (MAT cells) that remain in a state of G0/G1. In subculture, MAT cells replenish the mother cell line or give rise to new t-SRL variants. t-SRL lines are involved in pathogenesis and invasiveness. It is assumed that differentiated MAT cells mature to invasive hematophagic amoebae. ITD encystment seems to be absent in vivo [2].
The amplified fraction of mitoticly quiescent ISH cells (identical strict hypoxic cells) is the response of the t-SRL line to strict hypoxia Starting in 3xOCB culture sediments, the t-SRL line finished the current cell cycle by a symmetric cell division [8] and arrested 48 hrs after start as a ISH cell population paused in a G2 (G2/M) cell state. When transferred in AaEM encystment medium this G2/M population performs a new symmetric division and form ITD cysts in a ratio 1:2. If passage in fresh culture medium the ISH cell fraction restores the asymmetric cell fate, divides by asymmetric division and proliferates as an amplified new t-SRL line.
Responsible for reduced hypoxic proliferation of mammalian stem cells are several cell cycle inhibitor proteins and the hypoxia inducible factor HIF-1ά [9,10]. In multiple cell types, one of the major functions of HIF-1ά is the induction of metabolic reprogramming [11-13], namely from the catabolic metabolism pathway of the quiescent state to the anabolic metabolism of the activated cell state [8]. We do not have information in Entamoeba concerning the molecular metabolic differences that exist between s-SRL and t-SRL or respectively between the more oxygenic and the more hypoxic cell types.
However, it is evident that Entamoeba’s cell lineage development and life cycle depend on the oxygen gradients of its environment. Upper O2 zones induced the conversion of the primary p-SRL into the oxygenic s-SRL that proliferates by fast cycling. Lower oxygen contents induced P/T and S/T conversion. The t-SRL line proliferates after conversion by slow cycling. Multi- lined populations containing both s-SRL and t-SRL exist both in cecum and OCB cultures and both cell lines switch back and forth between fast and slow cycling. Hypoxia ≤ 1% O2 switches the t-SRL line to symmetric cell fate. ISH cells are quiescent identical cells arrested in a post- replicative G2 or G2/M state. Relief from hypoxia by oxygenation/ aeration restores the asymmetric cell fate, switching ISH cells into a amplified t-SRL line that proliferates in more hypoxic regions by slow cycling and in more oxygenic environments by fast cycling.
There are many hypoxia tolerant species and “facultative” anaerobes developing hypoxia defence mechanisms. Many authors consider that cellular level responses to oxygen lack may be relatively conservative in evolution and thus similar in different lineages. Cells may activate or silence batteries of genes for longer term backup defence against extended O2 limitation [14]. Entamoeba mainly responds to the natural oxygen in its environment by cell type conversion and genomic reprogramming. However, what happens with mitotic quiescent/mitotic arrested cells in the colon in conditions of anoxia?
MAS and MAT cells are basically different [2,7,15]. While MAS cells stop definitively the vegetative way of life and form in nutrient rich media ATD cysts, mitotic quiescent MAT cells remain vegetative continuing proliferation after pasaging. In nutrient free media they are capable to produce ITD cysts. In the colon, MAT cells migrate distally and are part of the population that colonizes the ascending, transverse and sigmo-rectal colon, forming ulcers. In vivo, MAT cells do not find conditions to form ITD cysts; they are excreted as hematophagous cells dying in feces.
The fate of the committed MAS precursor cells that fall under hypoxia is less known. We ask the following questions: Do committed MAS cells support oxygen depletion? Do they continue development or wait for re-oxygenation? Do they convert to another proliferating cell type or could they re-enter the mitotic cell cycle? What can MAS cells tell us about the basics of terminal differentiation? Questions and answers are of imminent importance to understand development of the primitive amoebic stem cell lineage. We try to clarify some of these aspects.
In the present paper we search for genome differences, DNA content and polyploidy in multi-lined populations of E. invadens and MAS cells response to progressive oxygen depletion in cultures. Finally we discuss some aspects of asymmetric and symmetric cell fate in Entamoeba.
Material and methods
OCB culture sediments - The culture method was described in detail in previous papers [7,16]. Accordingly, E. invadens were cultured in oxygen consuming bacterial sediments consisting of metabolically repressed Aerobacter aerogenes covered by a culture medium preconditioned by Serratia marcescens. The standard OCB dose in cultures was 5 mg A. aerogenes. Persistent suppression of bacterial growth has been reached by adding a bacteriostatic mixture of streptomycin and erythromycin to the culture medium.
Amoebic inoculi - In the present study the OCB sediments were inoculated with high amoebic doses containing ≈ 2.5 × 106 amoebic cells. The cultures contained secondary and tertiary amoebic populations produced by secondary s-SRL and tertiary t-SRL cell lines.
Detection of MAS polyploids as immature precursor cells for ATD encystment - Immature 8C polyploid precursor not passing the checkpoint of terminal differentiation (TD checkpoint) were induced to form ITD cysts by a modified AaEM medium containing 400 mM hydroxyurea (EMHU medium). The basic AaEM medium for mass ITD encystment consists of the same hypoosmotic nutrient-free medium lacking hydroxyurea [7].
Encystment sediments - Culture sediments exposed to EMHU were enriched by additional OCB doses (2 × 5 mg). These enriched sediments of about ~3xOCB doses were covered by the hyposmotic EMHU lacking antibiotics. Nutrient-starved 3xOCB sediments submersed in antibiotic-free AaEM or EMHU also consume oxygen, however at a more reduced rate as in nutrient-rich cultures.
Short and long term EMHU treatment - Amoebic populations of different ages (3 – 20 hrs old) were exposed 16-20 hrs to EMHU. Short treatments lasted usually 1-3 hrs and the EMHU medium was replaced then by fresh culture medium containing antibiotics. These cultures quickly become hypoxic: the ~3xOCB sediments rapidly consume oxygen. Stirring for counting aerates the sediments and starts a new oxygenic growth phase.
Oxygen consumption in cultures with ≈ 2.5 × 106 amoebic cell and ~3xOCB doses - Large amoebic populations consume oxygen [7,16]. Oxygen consumption increased rapidly when cultures were started with abundant amoebic inoculi up to 10 times higher as minimal standard inoculum of 2.5 × 105 amoebic cells. Culture hypoxia could be reduced by aeration, medium substitution, bacteria supplementation and stirring.
Results
The first MAS cell generation in high density multi-lined amoebic cultures (culture age: 0 – 10 hrs)
In OCB cultures started with ≈ 2.5 × 106 amoebic cells and 5 mg OCB, the oxygen content of the bottom sediment is consumed faster than in cultures with standard amoebic inoculi [7]. Oxygen consumption by abundant amoebic population hinders and delays MAS cell development to ATD cysts.
The multi-lined t-SRL/s-SRL culture in the Table 1 contains a dominant cell subpopulation produced by the tertiary t-SRL line and a minor MAS cell fraction of about 1% (8.0 × 104 MAS cells, sample 1f) produced by the secondary s-SRL line. The dominant tertiary cell population of ~99% cells capable to encyst in AaEM medium lacking hydroxyurea do not contain endopolyploid cells and do not encysts in encystment medium containing hydroxyurea (EMHU). The EMHU check is a suitable instrument to detect hidden polyploids in cultures; only 8C polyploids containing 8 genome copies form cysts in encysting medium containing hydroxyurea.
Parallel cultures
(sample) |
Culture age
in min/hrs |
All cells in culture
x 106 |
Mature polyploid precursors forming ATD cysts in cultures (x 104) |
Mature
polyploids
(in%) |
Mature & immature
polyploids encysting in EMHU (x 104) |
All polyploid
cells in culture
in% |
|
t0 |
2.70 |
|
|
|
|
1a |
t250 / 4:10 |
5.44 |
|
|
|
|
1b |
t450 / 7:30 |
5.60 |
|
|
1.99 |
25 |
1c |
t490 / 8:10 |
5.77 |
0.22 |
2.75 |
|
|
1d |
t530 / 8:50 |
5.38 |
0.41 |
5.12 |
4.33 |
54 |
1e |
t570 / 9:30 |
5.67 |
0.65 |
8.12 |
|
|
1f |
t625 / 10:25 |
5.77 |
0.88
|
11.0 |
8.00 |
100 |
Table 1. Mature and immature polyploid precursor cells in the early growth phase (t0 – t 625 min).
A high amoebic inoculum of 2.70 × 106 amoebic cells was cultured in sediments containing 5 mg metabolically repressed OCB. The current cell cycle ends between 180 and 240/250 min by asymmetric/ asynchronous cell division. The s-SRL line produces a first generation of 8 × 104 MAS cells capable of endoreplication. Cells encysting in culture represent mature polyploid precursor cells capable to form ATD cysts in culture medium. Cells encysting in EMHU represents immature polyploid precursors respectively polyploid cells that needs for passaging the TD checkpoint a phase of maturation that can be performed in EMHU medium. EMHU, encystment medium containing 400 mM hydroxyurea; The EMHU check lasted 16 hrs.
After culture start, both cell lines finished the current cell cycle and divide by asymmetric cell division between t180* and t250* (sample 1a). The subsequent cell division did not occur before t625 (sample 1f). The MAS cells of the first cell generation differentiate in two cell fractions: (i) an early MAS cell fraction of about 11% (~ 0.88 × 104 cells) finishing endopolyploidisation between t402 and t428 and (ii) a late MAS cell fraction of about 88% (~ 7.12 × 104 cells) that need more time for endoreplication and finish it between t428 and t625.
Endopolyploidisation and polypoid cell maturation as distinct developmental phases prior encystment
Committed non polyploid MAS cells- 250 min after start the just borne MAS cells fraction generated by asymmetric division are not polyploid and therefore unable to produce cysts when transferred in EMHU. Polyploid 8C MAS cells capable to encyst in EMHU appeared in cultures until t400*[3]. First juvenile cysts appear from t447* (Figure 1).
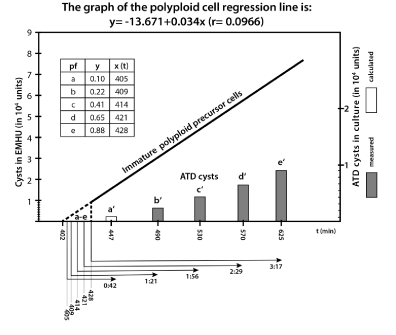
Figure 1. Oxygen depletion slow down MAS cells development to ATD cysts;
The regression line “Immature polyploid precursor cells” reveal the number of immature polyploids in culture (MAS cells) that mature and form ATD cysts in EMHU (measured values). The intermittent part of the regression line reveals five virtual polyploid fractions a - e (calculated data) that finished endoreplication in the time interval between t402 –t428 (see box) and form ATD cysts in culture between t447 and t625. b′- e′ bars correspond to the cyst number measured in the culture samples 1a-1f (see table 1), a′ is calculated from the corresponding regression line as a virtual 1% cyst fraction. The MAS subfraction “a” need about 4:45 hrs (t180-t447) to form juvenile cysts, a bit later borne subfraction “e” need at least 6:25 hrs, and still later borne MAS cells (≤ t250) need even much more time. t, time in minutes.
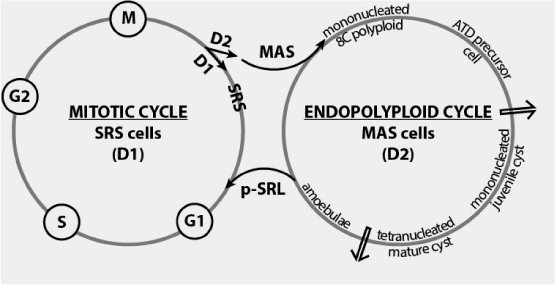
Figure 2. MAS cells: mitotic cell cycle exit and switching in the endopolyploid cycle. First arrow show the checkpoint for terminal differentiation (TD check point) [14].
The early MAS cell fraction (11% cells) - According to the data in Figure 1 the 11% MAS cell fraction (producing ATD cysts in high density amoebic cultures) finished genome amplification in rapid succession in about ~30 min (from t402 to t428). In our opinion these cells are descendants of cycling stem cells from the G2 or G2/M phase. Consequently, they enter asymmetric cell division synchronously and much earlier than the other 88% SRS cells. The early MAS cell fraction of about 11% needs approximately 220 min (3.6 hrs) to finish endoreplication (from t180 to t402).
We identify in this early MAS cell fraction five virtual subfractions a-e, which give rise in culture to the cyst fractions a′-e′. These virtual a-e subfractions contain polyploid precursor cells. In culture, before transiting the TD checkpoint, they complete the process of cell maturation.
Acute oxygen consumption occurring in culture up t420 hindered and delayed MAS cell development. The process of endopolyploidisation became asynchronous and requires over the the course of the experiment more and more time to complete.
First polyploid MAS cell subfraction finishing endopolyploidisatioin at t405 was the virtual subfraction “a” calculated from the regression line of the Figure 1. It needs ~0.45 hrs to encyst in culture, including cell maturation, check point transition and juvenile cyst wall formation (from t405 to t447) while the next cell subfractions “b-e” needed longer and longer namely: ~1:21 hrs (b/b′), ~ 1:56 hrs (c/c′), ~ 2:29 hrs (d/d′) and ~ 3:17 hrs (e/e′).
Referring to the moment of cell division the first borne MAS fraction “a” needed about 4:45 hrs (t180-t447) to form juvenile cysts, a later MAS cell fraction “e” need at least 1.5 hrs more (6:25 hrs) and even later MAS cells require significantly more time.
The data above suggests that 400 min after culture start oxygen depletion reached a critical level. Progressive hypoxia increase occurring between t400 and t625 delayed cell maturation preceding check point transition and cyst wall formation.
The late MAS cell fraction (88% cells) - require longer times to complete developmental processes preceding ATD encystment. The regression line of the Figure 1 shows the time period needed by this fraction for endopolyploidisation. The cells of this fraction need up to 625 min to end the endopolyploidisatioin phase, nevertheless all these polyploids are immature at t625. However, they are capable of finishing maturation in EMHU and encystment. In cultures with abundant amoebic populations endopolyploidisation and polyploid cell maturation are progressively asynchronous and requires over the ages more and more time.
The subsequent MAS generations (culture age: 10 – 20 hrs)
The data from the Table 2 show the development of the s-SRL line consisting of 1.18 × 104 cells during the subsequent period of growth (t10 –t20 in hrs). In this time period the s-SRL goes through two further cell divisions producing a total of three generation of MAS cells (D21-3 cells). One half of the first generation of MAS cells (0.55 × 104 D21 cells) completes maturation before t12, giving rise to juvenile ATD cysts in culture. The other half (0.63 x 104 cells) are immature precursors cells. They need a further 7 hrs to form juvenile cysts in cultures (sample 2b, 2c). The data are quite similar with the preceding findings concerning precursor cell maturation and terminal differentiation check point passage (TD check point) in high density amoebic cultures.
Table 2. Three generations of mature and immature precursor cells in culture and EMHU.
Sample |
Culture age
(hrs) |
ATD cysts in culture /
Mature polyploids
x 104 |
ATD cysts in EMHU / Mature & Immature polyploids
x 104 |
Immature precursor cells x 104
|
2a |
t12 |
0.55 (D21 : 55%) |
1.18 (D21: all) |
0.63 |
2b |
t15 |
0.55 (D21 : 55%) |
2.45 (D21-2: all) |
1.90 |
2c |
t19 |
1.00 (D21 : all) |
3.23 (D21-3: all) |
2.23 |
Culture conditions are the same as those of the table 1; EMHU check lasted 24 hrs. The s-SRL line consisting of 1.18 × 104 cells goes through three cell divisions producing three generations of asynchronous MAS precursor cells. Each MAS cell generation consists of 1.18 × 104 (D21) cells. One half of them encyst in culture before t12 (sample 2a), the other half needs more as 3 hrs for maturation, check point transition and juvenile cysts formation (sample 2b, 2c). The second and third generation of immature precursors do not encyst in culture before t19. t, time in hrs., D1 and D2, non identical daughter cells, D1 (SRS cells), D2 (MAS cells).
The second generation of MAS cells (1.27 × 104 D22 cells) finished endoreplication at t15. At this time the polyploid pool of 2.45 × 104 cells contains 0,63 × 104 immature cells of the first cell generation (D21 cells) and 1.27 × 104 immature precursor cells of the second D22 cell generation, together a total of 1.90 × 104 cells encysting in EMHU. At t19 the juvenile ATD cyst fraction formed in culture contains 1.00 × 104 units (sample 2c). This fraction represents the total of the first generation of MAS cells (D21 cells) that needed up to 15 hrs to encyst in culture (from t4 to t19). Precursors of the second and third generation (D22-3) are suppressed by unfavorable culture conditions. When transferred in EMHU they pass through the checkpoint and form 2.23 × 104 new ATD cysts.
MAS cell development in samples briefly exposed to EMHU and re-cultured in the 3xOCB/EMHU sediments
Culture samples in culture sediments with standard OCB dose of 5 mg A.aerogenes (1xOCB) were treated for 1-3 hrs in EMHU medium with additional doses of bacteria (~3xOCB) after 6, 7 and 9.5 hrs of growth and then re-cultured together with the 3xOCB /EMHU sediment in fresh culture medium containing antibiotics (Table 3). The ~3xOCB sediments consume oxygen more rapidly than the standard 1xOCB dose does. Stirring for counting aerates the sediments and creates new oxygenic conditions.
Table 3. Polyploid precursors of the 6-9.5 hrs old standard cultures exposed to short EMHU treatment and re-cultured in strong hypoxic conditions.
Samples |
Standard culture (1xOCB)
age in hrs |
ATD cysts in culture
|
Post EMHU culture. 1st count : 24 hrs (cysts in 104); [A] |
Post EMHU culture. 1st count : 48 hrs (cysts in 104); [B] |
Post EMHU culture. 2nd count : 48 hrs (cysts in 104); [A] |
3a |
t6 |
0 |
3.33 D21 : 46% |
4.00 D21 : 56% |
6.26 D21 : 87% |
3b |
t7 |
0 |
8.22 D21 |
7.33 D21 |
13.32 D21,2 |
3c |
t9.5 |
0 |
14.44 D21,2 |
13.55 D21,2 |
14.50 D21,2 |
The s-SRL stem cell line is of about 7.15 × 104 cells (mean value of 7 samples). Yellow: tubes with ~7.15 × 104 amoebic inoculi; green: tubes with <7.15 × 104 amoebic inoculi. Cultures were started with 1xOCB dose (5 mg). For EM-HU treatments sediments were supplemented by 10 mg OCB. Some of the re-cultured samples were firstly examined at 24 hrs after start [sample A] and the others 48 hrs after start [sample B]. Sample [A] was re-examined again 24 hrs later (second count). Counting includes homogenization and oxygenation of culture sediments. D21, D22: MAS cells of the first and second cell generation in %.
The results in the samples 3b and 3c reveal a s-SRL cell line of about 7.22/7.33 × 104 cells that proliferates and divides in the initial culture (before EMHU treatment)giving rise to two MAS cell fractions representing two successive D21, D22 generations of MAS cells for a total of 13.32/14.44 × 104 cells. Both cell lines continue development in EMHU and subsequent re-culture.
Sample 3c- Both D21 and D22 MAS cell fractions from the sample 3c (9,5 hrs old culture) formed cyst as observed 24 hrs after EMHU treatment. Hydroxyurea and ~3xOCB hypoxia do not hinder the formation of ATD cysts in the D21 and D22 fractions. The D21 fraction finished endoreplication (~3.7 hrs) and polyploid cell maturation (~3.2 hrs) during the period of 9.5 hrs initial growth (see Figure 1) and passed through the TD checkpoint either during EMHH treatment phase or after culture- restart. The second cell division takes place most likely in EMHU. The non-polyploid D22 fraction survives hydroxyurea treatment. It continues development (endoreplication, maturation, checkpoint transition and cyst formation) during the permissive oxygenic phase of the post EMHU culture.
Sample 3b- Even in the case of the sample 3b (7.0 hrs old culture) the EMHU treatment does not affect the development of the D21 and D22 MAS cell fractions. We consider that the D21 fraction from the previous culture at about t180 –t210 min end its 3.7 hrs long phase of endoreplication before passaging in EMHU; it continues maturation, checkpoint transition and cyst formation in EMHU and subsequent re-culture. So long as the ~3xOCB sediment remains oxygenic the s-SRL line proliferates and divide giving rise to a new D22 fraction. However, increasing hypoxia stops D22 cells from finishing development (ATD cyst formation). Only homogenisation for counting at t24 hrs - including aeration and oxygenation - stimulates waiting MAS cells to continue the developmental processes. At this time the mother s-SRL line was no longer present in culture. Strong hypoxia converted it into a tertiary t-SRL. A third generation of MAS cells (D23) therefore does not exist.
Sample 3a- 6 hrs after starting the initial culture (sample 3a) both SRS cells and MAS cells are in stages of replication respectively endoreplication. The s-SRL line is most affected by the subsequent EMHU treatment that destroyed this line completely. A D22 cell fraction was no longer produced. 46-56% of the MAS D21 cells produced cysts before re-culture hypoxia became unfavorable, ~30% need aeration and about 13% of the D21 cells are destroyed by EMHU.
Discussion
There are many single celled eukaryotes such as Sacharomyces and Caulobacter that divide by asymmetric cell division giving rise to differentiated progeny, however, the mother cells are not stem cells [17]. In contrast, Entamoeba has true primitive stem cells and a true stem cell lineage (protolineage). It consists of a primary stem cell line (p-SRL) and two progenitor cell lines (s-SRL and t-SRL) producing differentiated precursor cell types capable of terminal differentiation. The protolineage of Entamoeba conserves the basic mechanisms of stemness developed by the eukaryotic common ancestor [7].
ATD cysts formed in cultures easily ex-cyst in subsequent passages when they meet adequate stimuli such as promoting bacteria, nutrient resources and appropriate oxygenic pressure. Similarly, ATD cysts produced in the cecum may ex-cyst in the distal colon giving rise to new amoebic lineages and multi-lined populations that promote autoreinfection. Consequently, encystment and ex-cystment can no longer be considered as two separate developmental processes occurring in different hosts. They are rather two sides of a coin.
The primordial biological goal of the en-cystment-ex-cystment sequence that take place both in culture and intestine is totipotency recovery. During this developmental cycle cells of reduced potency such as MAS cells generate via encystment young totipotent amoebulae that give rise to immunologically uncompromised cell lines. In this way, Entamoeba reloads all genomicly encoded functions including virulence and invasiveness and produces multi-lined cell populations.
ATD encystment is not a process of cell defense against harmful growth conditions. It occurs in nutrient rich environments and optimal growth conditions as a preventive measure against colon anoxia (<0.15% O2) and air hyperoxia (21% O2); excreted non-encysted MAT cells (hematophagous cells) are killed by air hyperoxia.
Cyst excretion, dissemination and further infections are rather coincidental complishments in Entamoeba’s the life cycle. It reflects the capacity of ancestral free living eukaryotes to wait the return of appropriate growth conditions.
Committed MAS cells - MAS cells are precursors of ATD encystment produced by the progenitor s-SRL line that proliferates by fast cycling in the upper ranges of the oxygen gradient (<5.5% O2). Young borne MAS cells are irreversibly committed to form cysts by intrinsic mechanisms of terminal differentiation and maternal cell fate determinants. Committed MAS precursor cells are forced to perform a predetermined course of development (endoreplication, maturation, cyst formation). In optimal developmental conditions the first borne MAS cells needed about 4:27 hrs to form juvenile cysts (3:40 hrs for endoreplication and 0:47 for cyst wall formation).
Early not yet polyploid MAS precursor cells can not be induced to replenish the mother cell line - as MAT cells do - and are incapable of converting to any other amoebic cell type. After asymmetric cell division, young MAS cells pass a point of no return. They are committed for encystment. MAS cells withdraw from the mitotic cycle and form ATD cysts in nutrient rich environments. Unfavorable hypoxia temporarily interrupts MAS cell development (endopolyploidisation, polyploids maturation, passage through the TD check point). However, it can not abolish the MAS cells predetermination for autonomous terminal differentiation (ATD).
Time limited disconnection of the developmental progression by hypoxia mimetics, such as OCB sediments as described in the present paper, is a wide spread phenomenon in both lower and higher eukaryotic systems and could be observed in the differentiation of malignant gliomas [18]. Hypoxia inducible factors (HIF) mediate cell response to decreased oxygen availability [19,20]. The withdrawal of hypoxia and knockdown of HIF-1ά restores the differentiation capabilities of developmentally arrested cells. High oxygen availability leads to inhibition of HIF-1ά signaling [21]. Many studies of the role of hypoxia and HIF-1ά during development showed that hypoxia decreased cell differentiation ability in a HIF-1ά dependent manner [22-25].
What prevents ATD encystment in conditions of OCB hypoxia and what are the molecular mechanisms that exert this impact? It is clear that the progressive hypoxia occurring in cultures with the standard OCB dose and high amoebic inoculi (both partners in the rapid consumption of oxygen) as well as re-culturing in hypoxic ~3xOCB cultures repress the genes necessary for polyploidisation and polyploid cell maturation. Hypoxia stops immature precursor cells at different steps of development. Differentiation was prevented by making the cells hypoxic. However, the molecular control mechanisms are unknown.
In mammals several transcription factors and transcriptional regulators have been shown to be important for cell differentiation [26-29]. In human, bronchial epithelial cells (NHBE) cultured at an air-liquid interface differentiate normally in cilliate-cells however differentiation is inhibited when NHBE cells are cultured under submerged conditions. Submersion creates hypoxic conditions that prevent differentiation by blocking the gene expression program required for ciliogenesis [26].
The hypoxic signaling mechanism in stem cell maintenance is the HIF system. The major mediators of hypoxic response in mammalian are the transcription factors HIF-1ά and HIF-2ά. They bind to the constitutively expressed HIF-1β that regulates directly many genes of the hypoxia response system. The expression of the ά subunit is stabilized when oxygen concentration is <5%. This is the hypoxic signaling for stem cell maintenance. When the O2 concentration is >5% O2 the number HIF-1ά subunits decreases; one speaks of oxygen mediated HIF degradation and several enzymes as prolylhydroxilases PHD1-3 are activated by O2 leading to metabolic differences. High expression of HIFs has a functional importance for stem cell maintenance [29]; The authors of the excellent book “Anaerobiosis and Stemness” Zoran Ivanovic and Marija-Vlaski Lafarge review the current knowledge of the HIF/PHD system of in protists and consider the mediating oxygen sensing mechanisms as derived and conserved from the eukaryotic common ancestor.
MAT cells - MAT cells are quiescent non committed cells in a state of G0 (G1/G0). They are produced by the t-SRL cell line that proliferates in oxygenic environments by fast cycling and in mid to low hypoxic niches by slow cycling. In OCB cultures mitotic quiescent MAT cells are capable of growth and maturing to hematophagous- like cells [2]. As known, the capacity for extended growth is particular high in G1 arrested cells [30]. In vivo MAT cells become invasive cells. The mechanisms that specify invasive behaviour are poorly understood. The invasive cell fate frequently requires G1/G0-cell cycle arrest [31].
Passaged MAT cells exit quiescence. When transferred into subcultures with the standard 1xOCB sediment and nutrient rich medium MAT cells switch to cycling cells entering mitotic cell cycle (late G1 cells); they form a new t-SRL line that proliferates asymmetrically [7]. When transferred into ~3xOCB sediments by nutrients and serum deprivation and low/mid hypoxia, quiescent MAT cells enter the developmental endopolyploid cycle and form cysts.
With other words, at the early G1 level, a molecular switch decides if the cells enter the mitotic cell cycle or the alternative endopolyploid cell cycle. In nutrient rich environments cells produce mitogens that initiate production of factors promoting mitotic proliferation and asymmetric cell fate. In conditions of nutrient deficiency early G1 cells - regardless of their origin (SRT or MAT cells) - were committed to become precursors for ITD encystment.
ILH cells in AaEM encystment medium - Cycling SRT cells in a state of G2 respectively G2/M state (belonging to the t-SRL line) divide in low hypoxic encystment sediments (~3xOCB) submerged in AaEM encystment medium by symmetric differentiative division giving rise to identical low hypoxic daughter cells (ILH cells). ILH cells are committed for ITD encystment and form ITD cysts. The amplified ILH cell pool is “fully-committed” [29].
ISH cells in culture and encystment media - In the hypoxic cultures started with the triple 3xOCB dose, oxygen consumption and hypoxia progressively affect the proliferation of the passaged t-SRL line replenished in parts by MAT cells [7]. At the beginning, oxygen depletion does not hinder current cell division. However, cells divide by a symmetric not differentiative cell division that gives rise to identical cell progeny. It expands the pool of undifferentiated progenitor cells amplifying the t-SRL line. Fate-determining factors (transcription factors) are partitioned equally in both daughter cells (symmetric segregation). Progressively increasing hypoxia stops later cell cycle progression maintaining the undifferentiated cell progeny in the G2/G0 state, at least up to t48. These cells are ISH cells (identical strong hypoxic cells). Between t48 and t60 large parts of the 3xOCB sediments are consumed by amoebae and hypoxia declines. As a result ISH cells recover asymmetric proliferation forming again non identical daughter cells.
The undifferentiated mitoticly arrested G2/G0 state persists as long as ISH cells reside in strict hypoxic environments. Hypoxia inhibits cell line proliferation despite abundant nutrients. In contrast, ISH cells transferred to low hypoxic 3xOCB sediments, submersed in AaEM encystment medium lacking nutrients, maintain the undifferentiated symmetric cell fate and divide by symmetric cell division to identical ILH progeny (identical low hypoxic daughter cells). ILH cells are early G1 cells committed in AaEM encystment medium for ITD encystment [7].
The above results revealed the capacity of the t-SRL line to switch between asymmetric and symmetric cell division, a process also observed in mammalian progenitor cells [32]. However, E. invadens’ symmetric cell fate is rather a transient cell state and not a persistent state of “complete self-renewing”. In other words, extrinsic regulatory factors such as increasing hypoxia are responsible for the disruption of asymmetric cell fate in E. invadens. Switching in the symmetric cell fate seems to be a dysregulation of t-SRL line proliferation induced by hypoxia. Symmetric self-renewal is rare in OCB cultures of E. invadens but frequent in axenic cultures of E. histolytica dividing axenically by binary fission.
The developmental endopolyploid cell cycle - In our opinion, encystment belongs to a developmental cycle (endopolyploidisation-depolyploidisation cycle) opponent to the mitotic cell cycle. Endopolyploidisation is an alternative to mitosis: only committed MAS cells withdrawing mitotic cell cycle enter the endopolyploid cycle by intrinsic mechanisms of commitment. Not committed MAT cells must be induced experimentally to ITD cyst formation [7,33]. When transferred in encystment medium induced MAT cells exit mitotic quiescence (G0 state) and switch into the early G1 phase. All early G1 cells before RP point (restriction point of replication) can be induced to switch into the endopolyploid developmental cycle.
It is evident, that early G1 cells assess their surrounding. They decide, depending on the environment, whether to continue the mitotic cell cycle (in nutrient rich media) or to switch in endopolyploidisation (in nutrient deficient media). MAS cell commitment for encystment is a point of no return. Analogous endopolyploidisation/depolyploidisation cycles were described in mammalian cell systems and cancer [34].
This developmental cyc2021 Copyright OAT. All rights reservists of five distinct phases namely: (i) whole genome amplification (endoreplication), (ii) maturation of polyploids precursor cells, (iii) passaging through the TD checkpoint, (iv) terminal differentiation (encystment) and (v) de-differentiation (excystment). In the low/mid hypoxic conditions of the present study (Table 1 and Figure 1) the first MAS polyploids finished the phase of endopolyploidisation about 400 min (6:40 min) after culture innoculation. If the mother SRS cells cycled in a manner similar to the dominant t-SRL line of the culture, the first 11% MAS cells produced in culture at about t180/t205 need about 3:45 hrs* to finish endoreplication and 6:25 hrs** to form juvenile cysts. We consider the 11% MAS cell fraction as originating from mother SRS cells in the G2 or G2/M phase. The second 88% MAS cell fraction originate rather from G1 mother cells. These cells need more time for endoreplication and encystment namely about 6:25 hrs** to finish endoreplication and up to 15:00 hrs*** to form cysts.
Although the foregoing asynchronous cell division takes no more than 70 minutes (t180-t250) the 88% MAS cell fraction requires ~200 minutes more to finish endoreplication as compared to the 11% MAS cell fraction. The findings show that cultures with extremely rich amoebic inoculi consume quite early significant amounts of oxygen, specifically between t180 and t250; rapidly increasing hypoxia affects all developmental phases of the s-SRL line including asymmetric cell division, MAS cell endopolyploidisation and maturation and juvenile cyst wall formation; nevertheless, all MAS cells of the first generation will still have managed ATD encystment in culture while cells of the next MAS cell generations did not. In previous less hypoxic OCB cultures started with one-tenth of the current amoebic inoculum and 1xOCB dose each MAS cell generation (ca 4 to 5 generations) could encyst during the first 30 hrs of growth [16].
The data above clearly demonstrate that endoreplication and terminal differentiation are two distinct phases of the endopolyploid cycle of Entamoeba and not a unique process as considered by some researcher studying multi-celled eukaryote systems [34]. In Entamoeba, only mature polyploid precursor cells transit the check point of terminal differentiation. Immature precursors need a phase of maturation before going through the check point of terminal differentiation.
Recent findings in mammalian cells argue in favor of a unifying concept concerning developmentally programmed polyploidy [35,36]. Authors found in mammals at least eight cases in which terminal differentiation of progenitor cells lead to the formation of non proliferative mononucleated polyploids cells by mechanisms quite similar with the encystment pathway that leads Entamoeba’s MAT cells to ITD encystment.
One of the best known examples of polyploid terminal differentiation in mammalian stem cells is the differentiation of trophoblast stem cells (TS cells) to non-proliferating TG cells by multiple rounds of endocycles. TS cells are progenitor cells exiting mitotic cell cycle. They are programmed to differentiate into viable non proliferative mononucleated polyploid state [35]. Similarly with the MAT cells, TS cells enter the quiescent G0 (G1) phase but only when nutrients are lacking [36,37]. Loss of mitogen activation occurs when the FGF4 is absent; it results in the loss of CHK1 protein concomitant with the appearance of the p57 and p21 proteins. As a consequence, cells exit mitotic cycle, and enter terminal differentiation [35,36].
These findings shows that ITD encystment reflects common mechanisms of induced terminal differentiation occurring in the evolution upon the cell cycle exit, G0/G1 state and nutrient deficiency. As a response to the extrinsic stimuli of nutrient deficiency cells withdraw from the mitotic cell cycle and enter endopolyploidisation. Similarly with E. invadens mammalian TS cells conserve the ancestral mechanisms of induced terminal differentiation found in Entamoeba.
Conclusions
Pericellular oxygen concentration determines stem cell fate and development. Similar to the stem cell systems of mammalians and humans the primitive stem cell system of the metabolic anaerobe “micro-aerophile” Entamoeba proliferates and differentiates in an oxygen gradient extending between strong hypoxia and the upper physioxic zone of the gradient. It is the oxygen gradient of the host intestine extending between 0.15% and 5.5% intestinal O2 content that manages and controls Entamoeba’s development. Two progenitor cell lines share lineage work. The s-SRL line is the most oxygenic cell line living exclusively at the upper physioxic level (~5-5.5% O2). It differentiates committed MAS precursor cells for ATD encystment by intrinsic mechanisms of terminal differentiation and protects amoebic stem cell lineage against exposure to atmospheric oxygen by producing the impermeable cyst wall. It produces ATD cysts in oxygenic nutrient rich environments. Committed MAS precursor cells support hypoxia as developmental arrested immature precursor cells. However, the MAS cells continue the developmental cell cycle (endopolyploidazation, maturation, TD checkpoint transition, cyst wall synthese) only in conditions of re- oxygenation. Undoubtedly, the s-SRL is the more recent cell line in the evolution of the amoebic protolineage.
Quite interesting is the older t-SRL line that spans the complete environmental range and all functions of the amoebic stem cell lineage. In the upper oxygenic zones it cycled by fast cycling similarly with the s-SRL, however, its differentiated MAT cells are not committed. MAT cells differentiate terminally to ITD cysts by extrinsic inducers or regenerate new t-SRL lines, also participating in tissue invasiveness. Lower oxygenic niches reduce t-SRL proliferation while strong hypoxia stops it and converts its descendants into a transitory non proliferating ISH state (hypoxic undifferentiated cell type) that amplify the t-SRL line by complete self-renewal. Switching from symmetric to asymmetric cell state is controlled by hypoxia. Reduction of strong hypoxia (O2 content increase) is needed to restore proliferation efficiency of the t-SRL line. The molecular mechanisms of the lineage are mostly unknown however the HIF system may play a crucial role. It would be expected that the hypoxic stability of HIF-1ά decreases the differentiation capacity of MAT cells; increasing oxygen pressure leads to HIF-1ά degradation reactivating differentiation. We hope, molecular biologists will take interest in this early stem cell protolineage conserved by Entamoeba and help to clarify the mechanisms that control it.
Acknowledgement
The author expresses his gratitude to Dr. Dennis Thomas (native English speaker) for reading of the manuscript and excellent support.
1Hypoxic areas: The oxygenic availability scale of Entamoeba could be divide in following zones: (i) strict hypoxic, (ii) low/mid hypoxic, (iii) low/mid oxygenic, (iv) upper oxygenic zone. They correspond in about to the oxygen zones of hypoxia, low- , mid- and upper physioxia as described by mammalian cells researchers [4].
[2]culture age in minutes (t)
[3]calculated time points by the regression line
References
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7: 150-161. [Crossref]
- Niculescu V (2016) Pathogenicity of Entamoeba depends on stem cell conversion, genome reprogramming and epigenetic gene regulation.
- Niculescu VF (2014) The evolutionary history of eukaryotes: how the ancestral proto-lineage conserved in hypoxic eukaryotes led to protist pathogenicity. Microbiology Discovery 2:3.
- Timpano S, Uniacke J (2016) Human cells cultured under physiological oxygen utilize two cap-binding proteins to recruit distinct mRNAs for translation. JBC: M116.717363.
- Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102: 4783-4788. [Crossref]
- Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, et al. (2014) Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota in humans and mice. Gastroenterology 147: 1055–1063.e8. [Crossref]
- Niculescu VF (2015) The stem cell biology of the protist pathogen Entamoeba invadens in the context of eukaryotic stem cell evolution. Stem Cell Biol Res 2: 2.
- Pearce EL (2010) Metabolism in T cell activation and differentiation. Curr Opin Immunol 22: 314-320. [Crossref]
- McNamee EN, Johnson DK, Homann D, Clambey ET (2013) Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res 55: 58-70. [Crossref]
- Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, et al. (2003) Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 23: 359-369. [Crossref]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, et al. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299-3303. [Crossref]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78. [Crossref]
- Semenza GL. Regulation of Metabolism by Hypoxia-Inducible Factor 1 (2011) Cold Spring Harb Symp Quant Biol 76: 347–353. [Crossref]
- Hochachka PW (1997) Oxygen – a key regulatory metabolite in metabolic defense against hypoxia. Amer Zool 37: 595-603.
- Niculescu VF (2015) Axenic stress leads the minor stem cell line of Entamoeba histolytica to defective mitosis and aberrant reversible endopolyploidy. XVIII Symposium on Amebiasis, Campeche, Mexico.
- Niculescu VF (2013) Growth of Entamoeba invadens in sediments with metabolically repressed bacteria leads to multicellularity and redefinition of the amoebic cell system. Roum Arch Microbiol Immunol 72: 25-36. [Crossref]
- Inaba M, Yamashita YM (2012) Asymmetric stem cell division: precision for robustness. Cell Stem Cell 11: 461-469. [Crossref]
- Lu H, Li Y, Shu M, Tang J, Huang Y, et al. (2009) Hypoxia-inducible factor-1alpha blocks differentiation of malignant gliomas. FEBS J 276: 7291-7304. [Crossref]
- Semenza GL (1999) Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15: 551-578. [Crossref]
- Chan DA, Giaccia AJ (2007) Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev 26: 333-339. [Crossref]
- Hakim F, Kaitsuka T, Raeed JM, Wei FJ, Shiraki N et al. (2014) High Oxygen condition facilitates the differentiation of mouse and human pluripotent stem cells into pancreatic progenitors and insulin-producing cells. J Biol Chem 289: 9623-9638. [Crossref]
- Heinis M, Simon MT, Ilc K, Mazure NM, Pouyssegur J et al (2010) Oxygen tension regulates pancreatic ß-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 59:662-669. [Crossref]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, et al. (2005) Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9: 617-628. [Crossref]
- Lin Q, Lee YJ, Yun Z (2006) Differentiation arrest by hypoxia. J Biol Chem 281: 30678-30683. [Crossref]
- Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, et al. (2007) Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res 313: 1-9. [Crossref]
- Gerovac BJ, Valencia M, Baumlin N, Salathe M, Conner GE, et al. (2014) Submersion and hypoxia inhibit ciliated cell differentiation in a notch-dependent manner. Am J Respir Cell Mol Biol 51: 516-525. [Crossref]
- Chen J, Knowles HJ, Hebert JL, Hackett BP (1998) Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left–right asymmetry. J Clin Invest 102: 1077–1082.
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD (2000) Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 23: 45-51. [Crossref]
- Ivanovic Z, Vlaski-Lafarge M (2016) Anaerobiosis and Stemness: An evolutionary paradigm. Academic Press. ISBN: 978-0-12-800540-8.
- Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, et al. (2015) Invasive Cell Fate Requires G1 Cell-Cycle Arrest and Histone Deacetylase-Mediated Changes in Gene Expression. Dev Cell 35: 162-174. [Crossref]
- Doupe DP, Alcolea, MP, Roshan A, Zhang G, Klein AM et al. (2012) A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337: 1091–1093. [Crossref]
- Niculescu VF (2016) Developmental and non-developmental polyploidy in xenic and axenic cultured stem cell lines of Entamoeba invadens and E. histolytica. Insights Stem Cells 2016, 2:1.
- Erenpreisa J, Cragg MS (2007) Cancer: a matter of life cycle? Cell Biol Int 31: 1507-1510. [Crossref]
- Ullah Z, Lee CY, Depamphilis ML (2009) Cip/Kip cyclin-dependent protein kinase inhibitors and the road to polyploidy. Cell Div 4: 10. [Crossref]
- Ullah Z, de Renty C, DePamphilis ML (2011) Checkpoint kinase 1 prevents cell cycle exit linked to terminal cell differentiation. Mol Cell Biol 31: 4129-4143. [Crossref]
- Zetterberg A, Larsson O (1985) Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. PNAS 82:5365–5369.


