Introduction: It is well established that benzodiazepines and their related agonists at the benzodiazepine site of GABA receptor present anxiolytic and amnesic properties, whereas β-carboline alkaloids exert anxiogenic and learning-enhancing actions. The goal of the present study was to investigate the therapeutic efficacy of Norharmane (NO) as a benzodiazepine receptor inverse agonist on learning and memory of the Streptozotocin (STZ) rat model of Alzheimer's disease (AD).
Methods: Eighty male Wistar rats were divided into control, STZ, Saline, STZ+ vehicle and STZ+ NO groups. For induction of AD, rats were administered with STZ (3 mg/kg) bilaterally into the lateral ventricles. Administration of either ethanol (0.2 ml) or NO (1, 2, 3, and 4 mg/kg, Intraperitoneal) started one weeks post-STZ injection on a daily basis for a duration of 10 days. Learning and memory performances of the rats were evaluated using Morris water maze (MWM) and shuttle-box, respectively.
Results: Escape latency was significantly increased in STZ groups compared to the control and saline groups (P<0.01). Low doses of NO (1 and 2 mg/kg) protected learning against STZ-induced impairment, whereas treatment with high doses of NO (3 and 4 mg/kg) led to further learning impairment in the STZ rat model of sporadic AD. The percentage of time spent, the swimming distance in the target quadrant and the step-through latency (STL) in the STZ+ NO (2 mg/kg) group were significantly higher than those in other STZ-induced AD groups (P<0.001).
Conclusion: The findings of the present study suggest that administration of low doses of NO (1 and 2 mg/kg) improves learning and memory, whereas high doses of it (3 and 4 mg/kg) worsened both learning and memory in the STZ rat model of AD.
There are several modulatory sites at GABA-A receptors which mediate the action of many drugs. It is well-established that agonists at the benzodiazepine site, of these receptor notably benzodiazepines, have anxiolytic and amnesic properties whereas β-carboline alkaloids as benzodiazepine receptor inverse agonist exert anxiogenic and learning-enhancing actions by antagonizing the action of benzodiazepines on GABA-A receptors [1-5]. A number of β-carboline alkaloids such as harmane (1-methyl-9H-pyrido-[3,4-b]indole) (HA) 1-methyl-β-carboline), harmaline (1-methyl-7-methoxy-3, 4-dihydro-β-carboline) and NO (9H-pyrido[3,4-b] indole), exist naturally in plant derived food products (wheat, rice, corn, barley, soybeans, rye, grapes, mushrooms, and vinegar), plant derived beverages (wine, beer, whisky, brandy, and sake), and plant derived inhaled substances (tobacco) [6]. The β-carbolines HA, NO and harmaline also present endogenously in the brain, kidneys, liver and blood [7-8]. They have neurotoxic effects on benzodiazepine and Imidazoline receptors. Since high plasma levels of β-carbolines have been detected in heavy smokers [9], alcoholics [10], heroin-dependent humans [11], patients with essential tremor [12] or Parkinson's disease [13] they are presumed to be involved in development of CNS disorders. Β-carboline alkaloids increase monoamine neurotransmitters such as norepinephrine, dopamine and 5-HT levels in brain through inhibition of monoamine oxidase (MAO) activity [14,15]. They also modulate voltage-activated calcium, sodium and potassium channel currents [16,17]. β-carbolines are assumed to have neuroprotective properties as well as cytotoxic properties [18-20], excitation and euphoria [21,22], analgesic effects [23], anticancerous and antibiotic properties [24,25]. It is also suggested that β-carbolines in high doses are epileptogenic, in medium doses anxiogenic, while in low doses, improve learning and memory [26].
Gruss A et al. have demonstrated a stimulatory impact of NH on dopaminergic neurons in primary mesencephalic cultures through dendritic and synaptic proliferation and increase in dopamine level [27].
The present study was designated to investigate the effect of NO as a benzodiazepine receptor inverse agonist on STZ induced rat models of AD using MWM task and shuttle-box apparatus, respectively.
Animals
A total of 80 Adult male Wistar rats (Razi Institute, Karaj, Iran), weighing 200–300 g were used at the beginning of the study for MWM apparatus and Passive avoidance test, separately. Animals were kept in an animal house with a 12/12 h light–dark cycle and controlled temperature (22 ± 2 °C). Animals were housed in groups of 6 in Plexiglas cages with free access to food and tap water except during the limited periods of experiments. Each group consisted of eight rats and each rat was used once only and killed immediately after the experiment. Behavioral experiments were carried out during the light phase of the light/dark cycle (light on 07:00 am). Animals were divided into 8 experimental groups; control, saline, STZ, STZ+ vehicle (ethanol) and STZ+ NO groups. Rats in the vehicle and NO groups received ethanol (0.2 ml) or NO (1, 2, 3, and 4 mg/kg, i.p.) on a daily basis for one week after operation for a period of 10 days before training. For induction of AD, STZ (3 mg/kg, i.c.v., 10 μl each) was administered bilaterally into the lateral ventricles. In saline group, saline was injected into the lateral ventricles instead of STZ. Learning performance of the rats was evaluated using MWM and shuttle-box 24 h after the last NO or vehicle injection. All the experiments were executed in accordance with the "Guide for the Care and Use of Laboratory Animals" (National Institute of Health Publication No. 80-23, revised 1996) and were approved by the Research and Ethics Committee of Qazvin University of Medical Sciences. Norharman (9H-Pyrido [3, 4-b]-indole) hydrochlorideand STZ was purchased from SIGMA-ALDRICH Company and anesthetic drugs (ketamine and xylazine) are products of Alfasan Company, Holland.
Induction of experimental dementia of AD by i.c.v. administration of STZ in rats
Animals were first anesthetized with intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (10mg/kg). STZ (3 mg/kg, 10 μl/injection site) or saline (10 μl/injection site) was then injected bilaterally into the lateral ventricles by performing stereotaxic surgery and using a Hamilton syringe [27]. Following coordinates were used for i.c.v. injection: 0.8 mm posterior to the bregma, 1.5 mm lateral to the sagittal suture and 3.6 mm ventral from the surface of the brain (Paxinosand Watson, 1997). Meninges were carefully kept undamaged during the procedure. A Hamilton syringe with a cannula diameter of 0.3 mm was used for injection of 3 mg/kg STZ solution. STZ was dissolved in saline shortly before application and it was injected bilaterally into the brain ventricles. The cannula was left in situ for a further 5 min following injection to allow passive diffusion from the cannula tip and to minimize spread into the injection tract. The cannula was then slowly removed from the scalp, which was then closed with sutures. To determine the precise administration of STZ into the cerebral ventricles, 30% of the rats were injected with 5 μl of diluted potent blue dye prior to microscopical examination of their brains.
Assessment of spatial learning and memory using the MWM
After NO treatment for 10 days, the MWM tests were conducted to assess the learning and memory performance. The escape latency (s) and path length (cm) were analyzed in each trial and averaged over four trials for each rat. The frequency the rat reached the former placement of the platform as well as the time spent in the former platform quadrant were detected within 60 s.
The MWM was a black circular pool (140 cm in diameter and 60 cm high) that was filled with 22_C water to a depth of 25 cm. The MWM protocol was a stringent protocol of four trials per day for five consecutive days. During each trial, each rat was placed into the water at one of the four cardinal points of the compass (N, E, S, and W), which varied from trial to trial in a quasi-random order. The rat had to swim until it climbed onto the escape platform. Animals that failed to find the platform within the allocated time were gently guided to the platform. At the end of each trial, animals were allowed to stay on the platform for 20 s. The escape latency (platform search time) for each trial was recorded. After the last trial, the animal was towel dried and returned to the home cage. The platform was removed during the spatial probe test, which was performed 2 days after the last acquisition trial. The rats were allowed to swim for 60 s, and we recorded the latency to reach the platform location, the time spent swimming within a zone [i.e., a 20-cm radius that was centered either on the original training location (target zone) or on an equivalent location in the opposite quadrant (opposite zone)], and the proximity (the average distance in centimeters of rats from the center of the platform location across the 60-s test). The velocity of each rat was also calculated. The analysis of the latency to reach the platform location, and time spent within a specified radius (zone) are consistently more sensitive measures of the MWM probe test performance in terms of detecting group differences [28].
Passive avoidance performance (shuttle box)
To assess the memory retention of animals, a passive avoidance test was performed. In this task, the animal learns that a specific place should be avoided since it is associated with an aversive event. A decrease in step-through latency (STL, retention latency) indicates an impairment in memory in the PA task. The passive avoidance apparatus consisted of two light (Plexiglas) and dark (Black) compartments of the same size (20×20×30 cm3) separated by a door. The floor of the dark compartment (i.e. conditioning chamber) was made of stainless-steel bars (0.5 cm diameter) separated by a distance of 1 cm. Intermittent electric shocks (50 Hz, 3 s) of 1 mA intensity were delivered to the floor of the dark compartment by an isolated stimulator.
Inhibitory-avoidance training
The rats were allowed to become familiar with the laboratory environment 1 h before the training or testing sessions. All training and testing was carried out between 08:00 AM and 12:00 AM. Each animal was placed in the light compartment for 20 s, after which time, the door was raised and the duration the animal waited before crossing to the dark (shock) compartment was recorded as the latency. The animal was removed from the experiment when it waited for more than 180 s to cross to the other side. Once the animal completely crossed to the next compartment, the door was closed and a 1 mA foot shock was delivered for 3 s. The rat was then removed from the apparatus and 2 min later, the procedure was repeated. Training was terminated when the rat remained in the light compartment for 120 consecutive seconds. All the animals were trained with a maximum of two trials.
Retention test
Twenty-four hours after training, a retention test was performed to examine long-term memory. Each animal was placed in the light compartment for 20 s, the door was opened, and the latency for entering into the shock compartment (as described in the training session, all 4 paws in) was measured as STL. During these sessions, no foot shock was applied and the test session ended when the animal entered the shock compartment or remained in the light compartment for 600 s (criterion for retention) [29].
Place learning
Figure 1 display place learning of different experimental groups in the MWM. As expected, the average escape latency (The latency time to find the hidden platform), escape distance (the path length to find the platform) in searching for the hidden platform decreased with the increase in training days. In the control group and saline groups there was shorter average escape latency and escape distance compared to STZ group. In the control group and saline groups the percentages of the total time elapsed and distance swum in the target quadrant in the probe test were also relatively higher compared to STZ group, however, there was no significant different between control and saline groups groups. An i.c.v. injection of 3mg/kg STZ resulted in a significant decline in spatial learning, with longer latency and distance in search for the underwater platform in STZ and STZ+ Vehicle groups compared to control group. These results indicate that STZ could significantly impair spatial learning and memory in rats. The difference of the average escape latency and escape distance between the control and STZ groups was significant on all of the training days F=4.93, (p <0.001) and F=3.38, (p <0.01) and F= 2.78, (p <0.05). The results of the present study have also shown that treatment of the rats with low doses of NO (1, 2 mg/kg) protected spatial learning against impairment induced by STZ. As shown in figure 1 treatment with low doses of NO (1, 2 mg/kg), reversed the spatial learning and memory impairments induced by STZ. The average escape latency and average escape distance in search for the hidden platform were significantly decreased in the NO (1, 2 mg/kg) plus STZ group compared to the STZ group. Although the differences between the STZ, STZ+ Vehicle and control groups were significant on all the training days, no significant difference was observed between the control and NO (2 mg/kg) plus STZ group. On the other hand treatment with high doses of NO (3, 4 mg/kg) led to further impairment of spatial learning in the STZ rat model of sporadic AD. The escape latency and distance in search for the hidden platform in the STZ+NO (4 mg/kg) group rats were significantly higher than the control group, similarly to that observed in the STZ group (Figure 1). Furthermore, the results of this study have indicated that swimming speed was increased in the consecutive training days in all the treatment groups. However, there was no significant difference between the experimental groups. Moreover i.c.v. injection of STZ, also resulted in a significant decline in spatial memory, therefore, the percentages of the total time elapsed and distance swum in the target quadrant (the number of times of crossing platform) after removing the platform (probe test) were significantly decreased in the STZ group compared to the control and saline groups. In contrast, these parameters were increased in the NO (1, 2 mg/kg) plus STZ groups compared to the STZ group. These results have shown that STZ application markedly impaired spatial learning and memory of the rats. On the other hand, treatment with NO (1, 2 mg/kg) effectively protected spatial learning and memory against STZ-induced impairment. The percentage of the total time elapsed and the distance swum in the target quadrant were decreased in the NO (3, 4 mg/kg) plus STZ group compared to the control group. Furthermore, our results have shown that pre-training injection of low doses of NO (1 and 2 mg/kg) improved the Step-Through Latency of the passive avoidance test, however, high doses of NO (3, 4 mg/kg) attenuated memory retention in the STZ rat model of AD (Figure 2). The time spent in the light area before entering the dark area and the total time that the rats spent in the light compartment during the Passive Avoidance test in the control, saline and STZ+NO (2 mg/kg) groups were significantly longer than STZ group (P<0.001). As there were no significant different between the control and saline group and between the STZ and STZ+ Vehicle groups, and between the STZ+ NO (1, 3 mg/kg) and STZ+ Vehicle groups the results of the saline and STZ+ Vehicle and STZ+NO (1, 3 mg/kg) have not been shown.
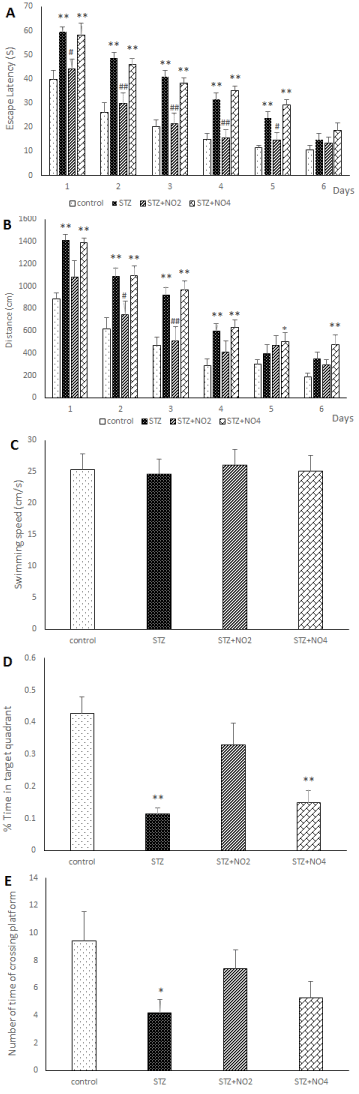
Figure 1. Effects of Norharmane (NO) on the escape latency (A) and the swimming distance (B) and the swimming speed (C) and the percentage of the total time elapsed in the target quadrant (D) and the number of times of crossing platform (F) in control and Experimental groups rats. The A panel shows the escape latency (the latency time to find the hidden platform). B panel shows the distance (the path length to find the platform) of the experimental groups during successive training days (four sessions per day). The D panel shows the percentage of time spent in the target quadrant. The F panel shows the number of times of crossing platform in control and Experimental groups rats during only one day (Probe test) in the experimental groups. *p<0.05; **p<0.001; relative to the control group, # p<0.05; ## p<0.001, relative to the STZ group, one-way repeated measure of ANOVA followed by the Tukey Post Hoc Test.
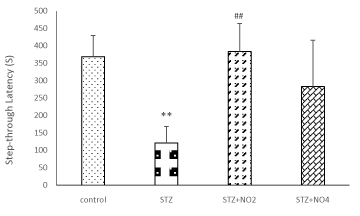
Figure 2. Comparison of STL during a Passive Avoidance test. Each value represents the mean± SEM of the latency to enter the dark compartment. (n=6 rat/group). Different doses of NO (1, 2 and 4 mg/kg, i.p.) or ethanol (0.2 ml, i.p.) were administered for 10 days before training in the four groups of animals. (**p<0.01) and (##p<0.01) indicate the difference from the control group and STZ + Vehicle group respectively.
In the present study, the effects of NO on learning and memory in the STZ-induced rat model of sporadic Alzheimer’s disease were examined. The main findings are as follows:
All parameters of place learning (escape latency, distance and number of crossed quadrants) in all the groups improved during the consecutive training days. An i.c.v. injection of 3mg/kg STZ significantly increased these parameters, in comparison with the control group. Pretreatment with low doses of NO (2 mg/kg) protected learning and memory against impairment induced by STZ, whereas pretreatment with high doses of NO (4 mg/kg) led to further impairment of learning and memory in the STZ rat model of sporadic AD. Also, our results show that the swimming speed of all groups of rats increased during the consecutive training days. However, there was no significant difference between experimental groups indicating that both STZ and NO had no effect on the motor activity of rats, so the effect of NO (2 mg/kg) did not improve the motor activity of rats. Therefore, NO probably attenuated STZ-induced neuronal damage in the brain.
Although our findings suggest that STZ disrupt spatial cognition, it is possible that the observed deficits in performance could have been a result of general behavioral or sensorimotor impairment, rather than a result of spatial learning and memory deficits. To investigate these possibilities, a visible platform task was performed. We found that STZ did not significantly affect the swim length to escape to the visible platform, a finding that is inconsistent with the idea that disruption of escape to the platform is due to general impairments.
In our experiments, spatial memory formation was measured by the percentage of time and distance swum in the target quadrant during a probe test. In the STZ group, these two parameters were significantly less than those in the control group. On the other hand, in the STZ + NO (2 mg/kg) group, these two parameters were close to those in healthy control rats so that there were no significant differences between these two groups, indicating that pretreatment with low doses of NO (2 mg/kg) attenuated STZ- induced impairment in memory. Our data suggested that administration of NO in low doses (2 mg/kg), improves learning and memory retrieval, whereas high doses of NO further worsen them in the STZ rat model of sporadic AD. In conclusion, the effect of NO on learning and memory is dose dependent. Confirming our results, Venault et al. has shown that low doses of β-carboline alkaloids improve learning and memory retention [26].
The capability of a Benzodiazepine receptor inverse agonist to enhance acquisition and/or consolidation processes has been established in several different memory tasks [30-32,4]. The dose of β-carboline required for enhancing memory is usually lower than the dose required for eliciting anxiogenic actions [5,26] and this rule was replicated in our study. Confirming our results, Samardˇzi´c et al. have shown that DMCM, a benzodiazepine site inverse agonist, at the dose of 0.1 mg/kg improves active avoidance in the rats [1].
Most of the benzodiazepines are known to produce both anterograde and retrograde amnesia [33-35]. In contrast, benzodiazepine receptor antagonists, such as flumazenil [34] and CGS 8216 [36], attenuate amnesia induced by various benzodiazepines. Similar results were obtained by β-carboline, such as FG7142 [37], beta-CCM, [30,38,39], NO [40], Ro 15-4513 [41] and harmine [42]. It has been demonstrated that NO reversed anterograde amnesia-induced by brotizolam in rats. Furthermore, it has been reported that flumazenil reversed anterograde amnesia-induced by benzodiazepines [35]. These results suggest that benzodiazepine-induced anterograde amnesia may be mediated through benzodiazepine receptors. On the other hand, retrograde amnesia induced by brotizolam is reversed by l-glutamic acid but not NO. It suggests that triazolobenzodiazepine-induced retrograde amnesia may be mediated through glutamate receptors.
One of the mechanisms through which NO exhibits anti-amnesic effects may be interaction with either benzodiazepine receptors or MAO, since NO can also bind to 5-HT as well as dopamine receptors and have Monoamine oxidases (MAO) inhibitory properties, [43,40].
It is assumed that any compounds that decreases the consciousness level (arousal level) interferes with learning and memory. Furthermore, any substance that increases the consciousness level improves learning and memory retention. According to this hypothesis, drugs like benzodiazepines that decrease the consciousness level lead to both anterograde and retrograde amnesia, while their receptor antagonists, such as flumazenil and benzodiazepine receptor inverse agonists, such as NO, that can increase the consciousness level recover the memory impairment which is induced by benzodiazepines [44-46]. Therefore, it can be suggested that NO, probably through increase the consciousness level can improve learning and memory retention in the STZ rat model of sporadic AD. In agreement with the findings of the present study, Gruss et al. have reported that chronic injection of 9-methyl-β-carboline (2µmol/100 g b.w.) for a period of 10 days improved spatial learning and stimulated synthesis of DA in hippocampus. It could increase DA, dendritic length/complexity and the number of dendritic spines in hippocampal formation [47,27,48]. Furthermore, it has been shown that 9-me-BC had protective and regenerative/restorative effects on dopaminergic neurons by inducing the gene expression of several neurotrophic factors and dow-regulating apoptotic cell signals.
Therefore, it can be suggested that low doses of NO, as a β-carboline similar to 9-me-BC, may have protective and regenerative/restorative effects on dopaminergic neurons that could be beneficial for the treatment of AD. The results of the present study have also shown that high doses of NO (3, 4mg/kg) decline the learning and memory retrieval in STZ-induced AD rat models. In agreement with these results, it has been shown that immediate intra peritoneal administration of HA (5 and 10 mg/kg) after training impaired learning and memory [49]. It has also been reported that administration of HA, to rat elicited visuo-motor, spatial learning and memory deficiencies in a dose-dependent manner [50]. Similar results were obtained when β-carbolines, such as HA and NO were administered [51,52]. It has been demonstrated that treatment with HA (2.5, 5, and 7.5 mg/kg, i.p. 30 min before each session of experiments, significantly decreased the retention latency in a dose dependant manner [51].
Previous studies have reported the involvement of cholinergic, dopaminergic and serotonergic systems in β-carbolines, such as HA -induced amnesia in the step-down passive avoidance test [49,53]. Furthermore, learning and memory may be impaired due to the major effects of β-carbolines on inhibition of MAOA and MAOB [54], and the elevated level of dopamine in the synaptic clefs. Another possible mechanism of memory impairment induced by high doses of NO might that triggered by the decreased level of nitric oxide in the brain. It has been demonstrated by Yoon et al. that β-carboline alkaloids decrease the level of inducible nitric oxide synthase (NOS) protein and NOS promoter activities. nitric oxide signaling via cGMP plays an important role in neuronal growth and synaptic remodeling after axotomy [55-57]. It is presumed that NO in high doses disrupts nitric oxide synthase and consequently impairs learning and memory. Further studies are required to elucidate the molecular mechanism of NO on CNS and its therapeutic effect on AD.
In conclusion, our results suggest that low doses of NO improve learning and memory processes, therefore, it can be used as a potential treatment of AD. However, high doses of NO further worsening of learning and memory in the STZ rat model of AD.
This research was funded by Qazvine University of Medical Sciences.
The Ethics Committee of Qazvine University of Medical Scinces approved the study.
- Samardžic J, Strac DŠ, Obradovic M,Opric D, Obradovic DI. (2012) DMCM, abenzodiazepine site inverse agonist,improves active avoidance and motivation in the rat. Behav Brain Res 235: 195–199. [Crossref]
- Korpi ER, Gründer G, Lüddens H (2002) Drug interactions at GABA(A) receptors. Prog Neurobiol 67: 113-159. [Crossref]
- Chebib M, Johnston GA. (2000) GABAactivated ligand gated ion channels: medicinalchemistry and molecular biology. J Med Chem 43:1427–47. [Crossref]
- Krazem A, Borde N, Beracochea D. (2001) Effects of diazepam and beta-CCM on working memory in mice: relationships with emotional reactivity. Pharmacol Biochem Behav 68 :235–244. [Crossref]
- Chapouthier G, Venault P (2002) GABA-A receptor complex and memory processes. Curr Top Med Chem 2: 841-851. [Crossref]
- Adachi J, Mizoi Y, Naito T, Yamamoto K, Fujiwara S, et al. (1991) Determination of beta-carbolines in foodstuffs by high-performance liquid chromatography and high-performance liquid chromatography-mass spectrometry. J Chromatogr 538: 331-339. [Crossref]
- Hudson A.L, Gough R. (1999) Novel selective compounds for the investigation of Imidazoline receptors. Ann N Y Acad Sci 881: 81–91. [Crossref]
- May T, Greube A. (12021 Copyright OAT. All rights reservng characteristics of the beta-carbolines harman and norharman in rat brain and liver and in bovine adrenal medulla. Naunyn Schmiedebergs Arch. Pharmacol 349: 308–317. [Crossref]
- Spijkerman R, van den Eijnden R, van de Mheen D, Bongers I, Fekkes D (2002) The impact of smoking and drinking on plasma levels of norharman. Eur Neuropsychopharmacol 12: 61-71. [Crossref]
- Rommelspacher H, May T. (1991) Beta-carbolines and tetrahydroisoquinolines: detection and function in mammals. Planta Med 57: S85–S92. [Crossref]
- Stohler R, Hug I. (1996) Initial results with withdrawal treatments of male and female participants in the diversified Janus opiate prescription project in Basel. Praxis 85: 1537–1541. [Crossref]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, et al. (2002) Elevation of blood beta-carboline alkaloids in essential tremor. Neurology 59: 1940-1944. [Crossref]
- Kuhn W, Müller T, Gerlach M, Sofic E, Fuchs G, et al. (1996) Depression in Parkinson's disease: biogenic amines in CSF of "de novo" patients. J Neural Transm (Vienna) 103: 1441-1445. [Crossref]
- Adell A, Biggs TA, Myers RD (1996) Action of harman (1-methyl-beta-carboline) on the brain: body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology 35: 1101-1107. [Crossref]
- Rommelspacher H, May T, Salewski B (1994) Harman (1-methyl-beta-carboline) is a natural inhibitor of monoamine oxidase type A in rats. Eur J Pharmacol 252: 51-59. [Crossref]
- Collins MA, Neafsey EJ. (2002) Potential neurotoxic “agents provocateurs” in Parkinson’s disease. Neurotoxicol Teratol 24: 571-577. [Crossref]
- Splettstoesser F, Bonnet U, Wiemann M, Bingmann D, Büsselberg D (2005) Modulation of voltage-gated channel currents by harmaline and harmane. Br J Pharmacol 144: 52-58. [Crossref]
- Moura DJ, Rorig C, Vieira DL, Henriques JA, Roesler R, et al. (2006) Effects of beta-carboline alkaloids on the object recognition task in mice. Life Sci 79: 2099-2104. [Crossref]
- Balon M, Munoz MA. (1999) A fluorescence study of the molecular interactions of harmane with the nucleobases, their nucleosides and mononucleotides. Biophys Chem 80: 41-52. [Crossref]
- Muñoz MA1, Guardado P, Galán M, Carmona C, Balón M (2000) A spectroscopic study of the molecular interactions of harmane with pyrimidine and other diazines. Biophys Chem 83: 101-109. [Crossref]
- Rommelspacher H, Strauss S, Lindemann J (1980) Excretion of tetrahydroharmane and harmane into the urine of man and rat after a load with ethanol. FEBS Lett 109: 209-212. [Crossref]
- Ergene E, Schoener EP (1993) Effects of harmane (1-methyl-beta-carboline) on neurons in the nucleus accumbens of the rat. Pharmacol Biochem Behav 44: 951-957. [Crossref]
- Nenaah, G. (2010) Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia 81: 779-782. [Crossref]
- Martin L, Martin MA, del Castillo B, (1997) Changes in acid-base equilibria of harmine and harmane inclusion complexes with cyclodextrins. Biomed Chromatogr 11: 87-8. [Crossref]
- Hamsa TP, Kuttan G (2010) Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol 649: 64-73. [Crossref]
- Venault P, Chapouthier G (2007) From the behavioral pharmacology of beta-carbolines to seizures, anxiety, and memory. ScientificWorldJournal 7: 204-223. [Crossref]
- Gruss M, Appenroth D, Flubacher A (2012) 9-Methyl-ß-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendriticand synaptic proliferation. J Neurochem 121: 924-31. [Crossref]
- Monisha S, Gupta YK (2001) Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci 68: 1021–1029. [Crossref]
- Shafiee SM, Vafaeiy AA, (2016) Rashidy-pour A. effects of maternal hypothyroidism during pregnancy on learning, memory and hippocampal bdnf in rat pups:beneficial effects of exercise. Neuroscience 329: 151–161. [Crossref]
- Pourmotabbed A, Nedaei SE, Cheraghi M, Moradian S, Touhidi A, et al. (2011) Effect of prenatal pentylenetetrazol-induced kindling on learning and memory of male offspring. Neuroscience 172: 205-11.
- Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, et al. (1986) Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature 321: 864–866. [Crossref]
- File SE, Pellow S (1988) Low and high doses of benzodiazepine receptor inverse agonists respectively improve and impair performance in passive avoidance but do not affect habituation. Behav Brain Res 30: 31–36. [Crossref]
- Raffalli-Sebille MJ, Chapouthier G, Venault P, Dodd RH (1990) Methyl beta-carboline-3-carboxylate enhances performance in a multiple-trial learning task in mice. Pharmacol Biochem Behav 35: 281–284. [Crossref]
- Hung DZ, Tsai WJ, Deng JF (1992) Anterograde amnesia in triazolam overdose despite flumazenil treatment: a case report. Hum Exp Toxicol 11: 289-290. [Crossref]
- Singh N, Sharma A, Singh M (1998) Possible mechanism of alprazolam-induced amnesia in mice. Pharmacology 56: 46-50. [Crossref]
- Saraf MK, Kishore K, Thomas KM, Sharma A, Singh M. (2003) Role of platelet activating factor in triazolobenzodiazepines-induced retrograde amnesia. Behav Brain Res 142: 31–40. [Crossref]
- Cain DP (1997) Prior non-spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology (Berl) 130: 313–319. [Crossre]
- Leidenheimer NJ, Schechter MD. Evidence for noradrenergic involvement in mediating the FG 7142 discriminative stimulus. Pharmacol Biochem Behav 1992; 43:77–83.
- Venault P1, Chapouthier G, Simiand J, Dodd RH, Rossier J (1987) Enhancement of performance by methyl beta-carboline-3-carboxylate, in learning and memory tasks. Brain Res Bull 19: 365-370. [crossref]
- Jensen LH1, Stephens DN, Sarter M, Petersen EN (1987) Bidirectional effects of beta-carbolines and benzodiazepines on cognitive processes. Brain Res Bull 19: 359-364. [crossref]
- Anand A, Saraf MK, Prabhakar S. (2007) Sustained inhibition of brotizolam induced anterograde amnesia byNHand retrograde amnesia by L-glutamic acid in mice. Behav Brain Res 182: 12-20.
- Nabeshima T, Tohyama K, Kameyama T. (1988) Reversal of alcohol-induced amnesia by the benzodiazepine inverse agonist Ro 15-4513. Eur J Pharmacol 155: 211–217. [Crossref]
- He D, Wu H, Wei Y, Liu W, Huang F, et al. (2015) Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur J Pharmacol 768: 96-107. [Crossref]
- Ji JZ, Zhang XH, Li BM (2003) Deficient spatial memory induced by blockade of beta-adrenoceptors in the hippocampal CA1 region. Behav Neurosci 117: 1378-1384. [Crossref]
- Mejo SL (1992) Anterograde amnesia linked to benzodiazepines. Nurse Pract 17: 44, 49-50. [Crossref]
- Rinehart JB, Baker B, Raphael D (2012) Postoperative global amnesia reversed with flumazenil. Neurologist 18: 216-218. [Crossref]
- Hogan JB, Hodges DB Jr, Lelas S, Gilligan PJ, McElroy JF, et al. (2005) Effects of CRF receptor antagonists and benzodiazepines in the Morris water maze and delayed non-matching to position tests. Psychopharmacology (Berl) 2005; 178: 410-419. [Crossref]
- Polanski W, Enzensperger C, Reichmann H, Gille G (2010) The exceptional properties of 9-methyl-beta-carboline: stimulation, protection and regeneration of dopaminergic neurons coupled with anti-inflammatory effects. J. Neurochem 113: 1659–1675. [Crossref]
- Polanski W, Reichmann H, Gille G (2011) Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: a new anti-Parkinson drug? Expert Rev Neurother 11: 845-860. [Crossref]
- Nasehi M, Piri M, Nouri M, Farzin D, Nayer-Nouri T, et al. (2010) Involvement of dopamine D1/D2 receptors on harmane-induced amnesia in the step-down passive avoidance test. Eur J Pharmacol 634: 77-83. [Crossref]
- Meignin C, Hilber P, Caston J. (1999) Influence of stimulation of the olivocerebellar pathway by harmaline on spatial learning in the rat. Brain Research 824: 277–283. [Crossref]
- Celikyurt IK, Utkan T, Gocmez SS, Hudson A, Aricioglu F (2013) Effect of harmane, an endogenous ß-carboline, on learning and memory in rats. Pharmacol Biochem Behav 103: 666–671. [Crossref]
- Ostergren A, Fredriksson A, Brittebo EB (2006) Norharman-induced motoric impairment in mice: neurodegeneration and glial activation in substantia nigra. J Neural Transm (Vienna) 113: 313-329. [Crossref]
- Nasehi M, Sharifi S, Zarrindast MR (2012) Involvement of the cholinergic system of CA1 on harmane-induced amnesia in the step-down passive avoidance test. J Psychopharmacol 26: 1151-1161. [Crossref]
- Glennon RA, Dukat M, Grella B, Hong S, Costantino L (2000) Binding of beta-carbolines and related agents at serotonin (5-HT (2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol Depend 60: 121–132. [Crossref]
- Yoon JW, Kang JK, Lee KR, Lee HW, Han JW et al. (2005) Beta Carboline alkaloid suppresses NF-kappaB transcriptional activity through inhibition of IKK signaling pathway. J Toxicol Environ Health A 68: 2005-2017. [Crossref]
- Cooke RM, Mistry R, Challiss RA,Straub VA (2013) Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy. J Neurosci 33: 5626-37. [Crossref]


