There is currently no treatment consensus with regards to BK Nephropathy (BKN). Thus, balancing minimization of immunosuppression with the attendant risk of acute rejection remains paramount for long-term allograft survival.
Between Jan. 1, 2006 and Dec. 31, 2012, 371 adult renal transplants were performed at our center. Immunosuppression consisted of Thymoglobulin induction followed by maintenance therapy with Tacrolimus, Mycophenolate Mofetil (MMF) and either Sirolimus (88%) or prednisone (12%). Serum PCR screening for BK virus was obtained monthly. BK viremia was detected in 66 (18%) recipients (Group A) while the remaining 305 recipients were never detected to have viremia (Group B). Minimizing immunosuppression for Group A recipients occurred in a stepwise fashion and consisted of an immediate 50% reduction in MMF dosing following by complete discontinuation if viremia did not clear within 3 months. No MMF dosing adjustments were made in Group B patients. All recipients were followed long-term for patient survival and graft function and survival.
Group A patient survival at 1, 3, and 5 years was 100%, 98.5%, and 97% compared to 99.3%, 98.4%, and 95.4% in Group B. Graft survival at the same time intervals in Group A was 100%, 98.5%, and 93.9% versus 99%, 97.7%, and 92.5% in Group B recipients. Mean serum creatinine, mg/dl, levels at 1, 3, and 5 years in Group A were 1.6, 1.7 and 1.6 compared to 1.5, 1.5, and 1.6 in Group B. Subsequent to MMF dose reduction/elimination, only on Group A patient progressed to BKN (1.5%) and only 2 patients suffered from an episode of acute rejection (3%).
This pre-emptive reduction in immunotherapy at the detection of BK viremia was associated with a low rate of progression to BKN and the development of acute rejection. In addition to comparable long-term function.
BK virus; BK nephropathy; transplant outcomes
Abbreviations
BKN: BK Nephropathy; DBD: Donor after Brain Death; CMV: Cytomegalovirus; DCD: Donor after Cardiac Death; EBV: Epstein-Barr Virus; IS: Immunosuppression; MMF: Mycophenolate Mofetil; NKF KDOQI: National Kidney Foundation Kidney Disease Outcomes Quality Initiative; PCR: Polymerase Chain Reaction.
BK virus is a member of the Polymoviridae family. It was first isolated in 1970 from a kidney transplant recipient with a ureteric stricture [1]. Studies show that up to 90% of the general adult human populations become exposed to BK virus at some point, usually early in life [1-3]. While this is usually inconsequential in the immunocompetent patient, BK replication in immunocompromised patients can result in graft dysfunction and loss. The presence of virus in urine has been associated with transient graft dysfunction and is usually asymptomatic, however active serum replication of virus has been more closely associated with subsequent graft dysfunction and loss. Before the mid 1990’s, BK virus nephropathy was rarely identified as a clinical problem in renal transplantation. However specific pathological hallmarks ascribed to tissue invasive disease not only improved the detection and description of BK nephropathy, but led to alteration in post-transplant immunotherapy to minimize the damage [4]. Today, BK virus nephropathy is an important and emerging cause of renal transplant dysfunction and loss [2,3,5].
Currently, 10-20% of renal transplant recipients develop BK viremia, and 5–10% proceed to develop BK nephropathy [6-8]. Brennan et al prospectively evaluated the differences between viremia, viuria, and BK virus nephropathy with 3 different immunosuppressive regimens showing the direct relationship between immunosuppression and BK infection [4,9]. New immunosuppression and combination therapies are thought to be a contributing factor in the increasing prevalence of BK virus infection. However, improving assay quality as well as aggressiveness of monitoring could also result in increasing numbers of BK (+) patients [10-12].
The mainstay of treatment for BK viremia/nephropathy has been reduction of immunosuppression [8]. Unfortunately, the development of BK nephropathy carries with it a significant rate of graft dysfunction/loss [13,14]. With the concomitant risk of developing acute rejection, there has been considerable focus on the development of more sensitive and predictive screening methods [15,16]. PCR based detection has been the gold standard for screening serum and urine for BK infection [17]. At our center, we have also validated multiple PCR assays for BK screening [18].
Our center has developed significant experience in screening for BK viremia and subsequent nephropathy. Since 2006, we have utilized a monthly screening policy for all transplant recipients with concomitant 50% reduction in MMF dose with a positive result which we define as log3 copies/ml (1000 copies/ml) in 2 consecutive blood draws by real-time PCR [19]. Additionally, we found that monthly PCR monitoring for BK viremia, with reduction of immunosuppression resulted in not only improved results, but also decreased rates of cytomegalovirus- (CMV) and Epstein Bar Virus-related (EBV) disease, furthering the idea that BK viremia in addition to its pathologic effects can serve a surrogate marker for excessive immunosuppression [20].
In this study, we present long-term data on our screening and treatment protocol for BK viremia. Our aggressive posture towards detection has provided us a detailed view of outcomes associated with long term BK screening and its effects on graft and patient survival. Treatment of BK viremia with our screening and immunosuppression protocol was associated with a low rate of BKN as well as acute rejection. Additionally, long-term outcomes were comparable between patients with and without BK viremia over a 5-year period. This study demonstrates the effectiveness and safety of aggressive BK virus detection protocols.
Demographics
371 renal transplants were performed at our center between Jan. 1, 2006 and Dec. 31, 2012. The demographics of those patients who tested positive (BK+), as well as those remaining BK viremia free (BK-), representing the control group, are listed in Table 1.
Table 1. Patient Demographics
|
BKV+ (n=66) |
BKV- (n=271) |
p-value |
Mean Patient Age |
49.18 (15-73) |
47.64 (12-77) |
0.384 |
Male (%) |
72.73 |
59.67 |
0.048 |
DM (%) |
36.36 |
40.13 |
0.572 |
DDRT (%) |
71.21 |
71.15 |
0.992 |
1st Transplant |
90.91 |
87.5 |
0.439 |
Causes of ESRD |
|
|
|
DM |
6.06 |
10.82 |
0.242 |
HTN |
6.06 |
3.61 |
0.359 |
PCKD |
10.61 |
13.11 |
0.579 |
FSGS |
12.12 |
4.26 |
0.012 |
Toxin-Related |
1.52 |
2.95 |
0.514 |
Congenital |
1.52 |
0.98 |
0.705 |
IGA |
1.52 |
3.61 |
0.384 |
Other |
10.59 |
12.46 |
|
Unknown |
50 |
48.2 |
|
Immunosuppression Protocol
Our immunosuppression protocols have been describe previously (Table 2) [21]. Patients were induced with 4-5 mg/kg of Thymoglobulin depending on hematologic considerations and a Solumedrol taper (375 mg to 20 mg) over 6 days. Patients were maintained on Mycophenolate Mofetil (2g/day to 1g/day at day 6) and Tacrolimus (target level; 8-10 ng/ml on discharge). Sirolimus was initiated on post-operative day 6 targeting a level of 8-10 ng/ml 14 days following initiation. Maintenance immunosuppression consisted of Tacrolimus, Mycophenolate Mofetil (1 g/day), and Sirolimus (8-10 ng/ml). Initially, serum Tacrolimus levels were kept between 8-10 ng/ml, however 90 days’ post-transplant; Tacrolimus dose was reduced to achieve a 12hr trough level of 2 ng/ml. Post-transplant patients are followed in our clinic weekly and then monthly for the first five years following transplantation and then yearly after that.
Table 2. Immunosupression Protocol
Induction |
|
Thymoglobulin |
0.5-1.0 mg/kg/day for total dose of 4-5 mg/kg* |
Solu-medrol |
Day 0 - 375 mg, Day 1 - 200 mg, Day 2 - 160 mg, Day 3 - 120 mg, Day 4 - 80 mg, Day 5 - 40 mg, Day 6 - 20mg |
Tacrolimus |
8-10 ng/ml on Dischargea |
Mycophenolate Mofetil |
Day 0 - Day 5 - 2g/day, Day 6 - 1g/day |
Sirolimus |
Day 6 - 8-10ng/mlb |
Maintenance |
|
Tacrolimus |
Discharge - Day 90 - 8-10ng/ml, then 2ng/mla |
Mycophenolate Mofetil |
1 g/day |
Sirolimus |
8-10 ng/mlb |
*Dose reduced secondary to thrombocytopenia/leukopenia
a12 hr through
b24 hr through
BK Viremia Screening
BK viremia was identified as described previously [20]. Briefly, viral DNA was isolated from 100 mcL of plasma using NucliSens Magnetic Extraction Reagents (bioMerieux, Durham, North Carolina, USA) on an easy Mag extractor (bioMerieux) and eluted to a volume of 50 mcL. Hot-start PCR was performed using the primers described by Arthur et al. [16], which amplifies all polyoma viral DNA with a target sequence of 173 base pairs in the large T-antigen gene. The PEP-2 primer was biotin labeled to enable product detection. The equivalent of 20 mcL of sample was amplified in 50 mcL reactions containing 0.2 mM dNTP mixture, 10 mM Tris, 3 mM MgCl2, 25 pmol of each primer (Synthetic Genetics, San Diego, California, USA) and 1.25 U of Gold Taq (Applied Biosystems, Foster City, California, USA). All reactions were performed in a GeneAmp PCR System 9600 thermocycler (Perkin-Elmer, Norwalk, Connecticut, USA) with the following program: 1 cycle of 10 min at 95°C followed by 35 2-step cycles of 95°C for 15 s and 60°C for 60 s, followed by 5 min at 72°C. The PCR products were detected by enzyme-linked oligosorbant assays as previously described [22]. The DNA quantitation was based on optical densities from the amplification of plasmid DNA (3–6 log10 copies) engineered by cloning the target region (TOPO TA Cloning, Invitrogen, Carlsbad, California, USA).
Treatment Intervention
PCR BK serum concentrations at or above log3 copies/ml in two consecutive samples constitute a positive (+) viremia finding. Patients testing positive had their MMF dose immediately reduced by 50%. If the viremia failed to resolve within 3 months, the MMF was removed entirely. No dosing adjustments were made to Tacrolimus, Sirolimus, or steroid dosing. All patients continued to be screened monthly throughout the duration of the study period. Patients who showed signs of acute renal dysfunction, defined by a greater than 25% increase in serum creatinine, underwent renal biopsy for evaluation of rejection. All patients were followed long-term for patient survival, graft survival, and graft function.
Statistical Analyses
Descriptive statistics were used to summarize patient characteristics, as well as patient and graft outcomes. Statistical differences among patient demographics and causes of ESRD were calculated using Pearson’s Chi-square analysis. The two groups, BK+ and BK-, were compared using two-sample t tests with equal variances to determine statistical significance of differences in serum creatinine annually. P-values were two-tailed, considered statistically significant when <0.05, and determined using the Wilcoxon ranksum test. Patient and graft survival was assessed using Kaplan-Meier Survival curves and compared using Log-rank tests. All statistical analyses were performed using Stata v. 14.0 statistical software (StataCorp, College Station, TX).
Of the 371 patients transplanted between 2006 and 2012, 66 (18%) developed BK viremia. All 66 patients underwent reduction in MMF dosing. Only 1 patient in this subgroup progressed to BKN (1.5%) and only 2 experienced episodes of acute rejection (3%). No statistical difference existed between the groups with regards to mean age, percentage with diabetes (DM), percentage receiving cadaveric renal transplants (CRT), or percentage receiving their first kidney transplant (1st Transplant) (Table 1). The BK+ group contained a larger proportion of men than the BK- group (72.73% vs 59.67%, p=0.048). Differences between underlying causes of end-stage renal disease (ESRD) were not statistically significant except for the percentage with FSGS (p=0.012). However, approximately half of each group had either unknown causes of ESRD or causes that were unavailable at the time of data collection.
Patient Survival
Patient mortality was tracked using our electronic medical record with all-cause deaths recorded as occurring during a particular year after transplantation (Figure 1). The BK+ patients’ survival was 100%, 100%, 98.5%, 98.5% and 96.9% at years 1, 2, 3, 4, and 5, respectively. The BK- group had patient survivals of 99.3%, 98.4%, 98.0%, 96.4%, and 95.4% at the same time points, respectively. Although patient survival is slightly better in each year after transplantation in the BK+ group, no statistically significant difference exists (p=0.3037).
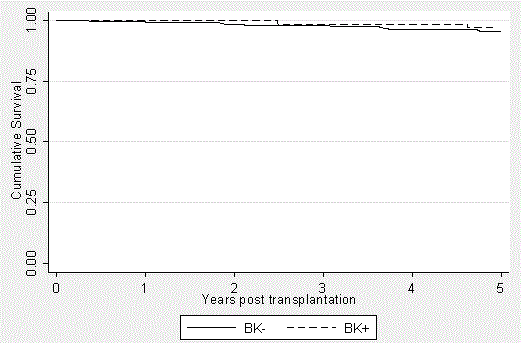
Figure 1. Patient Survival in the BK+ and BK- groups over a 5-year period posttransplantation. Kaplan-Meier curves were generated to express the very similar outcomes between the groups (p=0.3037), independent of BK viremia and subsequent immunosuppression modification. It should be noted that the patients transplanted in 2012 had not yet reached the five-year mark. This is accounted for in the data analysis.
Graft Function and Survival
Graft function was determined by serum creatinine levels on a yearly basis following transplantation (Figure 2). In cases where levels fluctuated around these time points, averages were noted after removing gross outliers. At the end of year 1, the mean creatinine in the BK positive group (n=66) was 1.61 ng/dl versus 1.46 ng/dl in the BK negative group (n=294; p=0.004). At the end of year 2, the BK positive group (n=64) had an average creatinine of 1.68 ng/dl while the BK negative group had an average creatinine of 1.50 ng/dl (n=276) was 1.50 (p=0.026). At the end of year 3, the BK positive group had a creatinine of 1.73 vs. 1.53 (n=58 vs. 2.62; p=0.005). Even though serum creatinine 4 years’ post-transplant were slightly different (1.74 ng/dl vs. 1.56 ng/ dl; p=0.065), serum creatinine 5 years after transplant were comparable (1.64ng/ dl vs. 1.58 ng/dl; p=0.211). Patients without serum creatinine measurements around the year-end period or lost to follow-up were excluded from the annual averages.
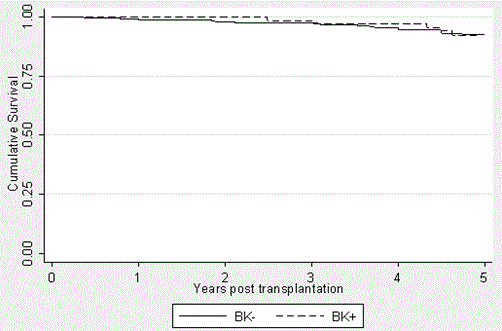
Figure 2. Graft Survival in the BK+ and BK- groups over a 5-year period posttransplantation. Kaplan-Meier curses were generated to express the similar graft outcomes (p=0.7794). Patient deaths were not censored and thus contributed to graft loss. It should be noted that the patients transplanted in 2012 had not yet reached the five-year mark. This is accounted for in the data analysis.
Graft survival (Figure 3) was defined as avoidance of dialysis and was not death censored. The BK+ group had graft survivals of 100%, 100%, 98.5%, 97%, and 92.3% at yeas 1, 2, 3, 4, and 5, respectively. This compares to survival in the BK- group of 99%, 98%, 97.4%, 94.8%, and 92.4% at the same time points, respectively. Again, no statistically significant difference was found to exist between these groups (p=0.7794).
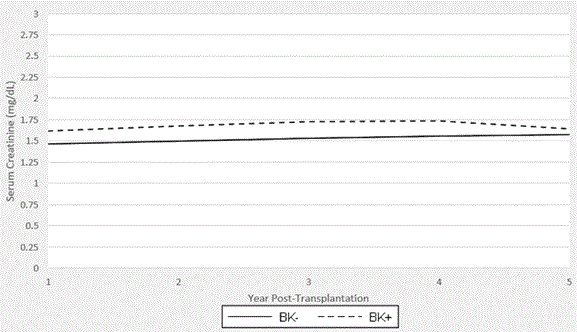
Figure 3. Graft Function in th2021 Copyright OAT. All rights reservriod post-transplantation. Average serum creatinine values per annum are shown. Years 1, 2, and 3 reveal higher levels in the BK+ group than the BK- group that reach statistical significance (year 1: 1.61 vs 1.46, p=0.004; year 2: 1.68 vs 1.50, p=0.026; year 3: 1.73 vs 1.53, p=0.005).Years 4 and 5 showed no statistically significant difference (year 4: 1.74 vs 1.56, p=0.065; year 5: 1.64 vs 1.58, p=0.211). It should be noted that BK seroconversion is most likely earlier in the post-transplantation period. The patients transplanted in 2012 had not yet reached the five-year mark. This is accounted for in the data analysis.
Polyoma viral infection after renal transplantation is a well described and feared complication with possible stepwise progression from viuria to viremia to nephropathy potentially leading to graft loss. If left unmanaged, graft failure will occur in one third of patients and decreased renal function in the residual cohort [23]. However, with PCR screening, BK viremia can be easily identified early in its course. When identified, the current clinical management entails reduction in immunosuppression, which can be associated with increased rates of acute rejection. No consensus currently exists with regards to modulating immunosuppression following diagnosis of BK viremia and/or BK Nephropathy [24]. This study provides a stepwise protocol for screening for and tapering immunosuppression in renal transplant recipients with BK viremia.
Current BK management protocols by National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) Guidelines recommend monthly BK testing with plasma nucleic acid testing for the first 3 months, then every three months for the first year and thereafter only if there is unexplained rise in serum creatinine or after an acute rejection episode with subsequent high dose immunosuppression (IS) therapy. Recommendations include decreasing immunosuppression when serum BK is >10,000 copies/mL [24,25]. At our center, we follow a screening regimen described by Koleilat et al. [20] screening patients monthly during the first year in an effort to improve early diagnosis [20]. In this study, BK viremia prevalence was 18% [20]. This is consistent with the described incidence of BK viremia in renal transplant recipients (10-20%; [8,23]) Importantly, we choose to intervene at plasma BKV loads of log3 or greater. This value was chosen as the lower limit of detection for a “positive” result using our assay. Current transplant center variance in screening (viuria vs viremia) as well as specific testing sensitivities make standardizing target numbers for therapy difficult, but we find improved accuracy with use of PCR screening of serum samples [26]. Our immunosuppression reduction strategy consisted of minimizing exposure to MMF, while making efforts to maintain Tacrolimus dose stable. However, center practices also vary, as other studies suggest that reduction of either Tac or anti-proliferative agents have similar overall viral load reduction to MMF reduction despite stronger association between Tacrolimus and BK viremia [27].
We document a favorable outcome for our BK+ group with a BKN rate of 1.5% and only 3% progression to acute rejection related to BKN. Importantly, with our immunosuppressive regimen this treatment intervention for BK viremia was associated with comparable long-term outcomes regarding graft function. Five-year graft survival was 93.9% in the BK+ group and 92.5% in the BK- group. To our knowledge, this is the longest follow up for a cohort of BK positive renal transplant patients.
While our protocol describes promising outcomes, the future of BK viremia management should focus on identifying risk factors and improving antiviral therapy. Thangaraju et al recognize specific risk factors (including extremes of age, male sex, depleting antibody, and HLA mismatch >4) which we may use going forward to identify high risk patients and potentially pre-emptively tailor immunosuppression to prevent development of BK viremia [28,29]. Other treatment strategies including cidofovir, leflunomide, IVIG and ciprofloxacin have been evaluated in many small series and appear to result in similar graft survival as IS reduction, although our practice is to use these as adjuncts to IS reduction with non-responding patients [30,31]. Randomized controlled trials are desperately needed to guide management and improve overall outcomes for BK+ renal transplant recipients. We will continue to follow our cohorts of patients in order to further define the best possible options for determining exposure to BK virus and improve therapeutic interventions.
The author(s) declare that they have no competing interests.
All Authors contributed equally to this work.
- Pinto M, Dobson S (2014) BK and JC virus: a review. J Infect 68 Suppl 1: S2-8. [Crossref]
- Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL (1980) Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med 92: 373-378. [Crossref]
- Gardner SD, MacKenzie EF, Smith C, Porter AA (1984) Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol 37: 578-586. [Crossref]
- Randhawa P, Brennan DC (2006) BK virus infection in transplant recipients: an overview and update. Am J Transplant 6: 2000-2005. [Crossref]
- Bohl DL, Brennan DC (2007) BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol 2 Suppl 1: S36-46. [Crossref]
- Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, et al. (2005) Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79: 1277-1286. [Crossref]
- Nickeleit V, Klimkait T, Binet IF, Dalquen P, Del Zenero V, et al. (2000) Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med 342: 1309-1315. [Crossref]
- Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, et al. (2005) Incidence of BK with Tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 5: 582-594. [Crossref]
- Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, et al. (2002) Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488-496. [Crossref]
- Mengel M, Marwedel M, Radermacher J, Eden G, Schwarz A, et al. (2003) Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant 18: 1190-1196. [Crossref]
- Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, et al. (1999) Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67: 918-922. [Crossref]
- Buehrig CK, Lager DJ, Stegall MD, Kreps MA, Kremers WK, et al. (2003) Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int 64: 665-673. [Crossref]
- Randhawa P, Ho A, Shapiro R, Vats A, Swalsky P, et al. (2004) Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol 42: 1176-1180. [Crossref]
- Wadei HM, Rule AD, Lewin M, Mahale AS, Khamash HA, et al. (2006) Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant 6: 1025-1032. [Crossref]
- Ahuja M, Cohen EP, Dayer AM, Kampalath B, Chang CC, et al. (2001) Polyoma virus infection after renal transplantation. Use of immunostaining as a guide to diagnosis. Transplantation 71: 896-899. [Crossref]
- Arthur RR, Dagostin S, Shah KV (1989) Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol 27: 1174-1179. [Crossref]
- Bechert CJ, Schnadig VJ, Payne DA, Dong J (2010) Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol 133: 242-250. [Crossref]
- Stellrecht KA, Espino AA, Nattanmai SM, Jackson WF, Conti DJ (2013) Comparison of three real-time PCR for the quantification of polyomavirus BK. J Clin Virol 56: 354-359. [Crossref]
- Petrov R, Elbahloul O, Gallichio MH, Stellrecht K, Conti DJ (2009) Monthly screening for polyoma virus eliminates BK nephropathy and preserves renal function. Surg Infect (Larchmt) 10: 85-90. [Crossref]
- Koleilat I, Kushnir L, Gallichio M, Conti DJ (2011) Initiation of a screening protocol for polyoma virus results in a decreased rate of opportunistic non-BK viral disease after renal transplantation. Transpl Infect Dis 13: 1-8. [Crossref]
- Lopez-Soler RI, Chan R, Martinolich J, Park L, Ata A, et al. (2017) Early steroid withdrawal results in improved patient and graft survival and lower risk of post-transplant cardiovascular risk profiles: A single-center 10-year experience. Clin Transplant 31: 2.
- Stellrecht KA, Woron AM, Mishrik NG, Venezia RA (2004) Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J Clin Microbiol 42: 1528-1533. [Crossref]
- Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice (2013) BK polyomavirus in solid organ transplantation. Am J Transplant 13 Suppl 4: 179-188. [Crossref]
- Pham PT, Schaenman J, Pham PC (2014) BK virus infection following kidney transplantation: an overview of risk factors, screening strategies, and therapeutic interventions. Curr Opin Organ Transplant 19: 401-412.
- Pham PT, Reddy UG (2013) Transplantation: Immunosuppression and risk of polyomavirus BK replication. Nat Rev Nephrol 9: 135-136. [Crossref]
- Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, et al. (2009) Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis 11: 1-10. [Crossref]
- Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, et al. (2010) Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10: 2615-2623. [Crossref]
- Thangaraju S, Gill J, Wright A, Dong J, Rose C, et al. (2016) Risk Factors for BK Polyoma Virus Treatment and Association of Treatment with Kidney Transplant Failure: Insights From a Paired Kidney Analysis. Transplantation 100: 854-861. [Crossref]
- Pai D, Mann DM, Malik A, Hoover DR, Fyfe B, et al. (2015) Risk Factors for the Development of BK Virus Nephropathy in Renal Transplant Recipients. Transplant Proc 47: 2465-2469. [Crossref]
- Kable K, Davies CD, O'connell PJ, Chapman JR, Nankivell BJ (2017) Clearance of BK Virus Nephropathy by Combination Antiviral Therapy With Intravenous Immunoglobulin. Transplant Direct 3: e142. [Crossref]
- Gonzalez S, Escobar-Serna DP, Suarez O, Benavides X, Escobar-Serna JF, et al. (2015) BK Virus Nephropathy in Kidney Transplantation: An Approach Proposal and Update on Risk Factors, Diagnosis, and Treatment. Transplant Proc 47: 1777-1785. [Crossref]



