The incidence of mosquito-borne diseases such as Zika, Chikungunya and Dengue is showing an upward trend in different geographical locations worldwide and has resulted in enormous tragic loss to human life [1,2]. Zika is a member of the virus family Flaviviridae, which spread mostly through the bite of daytime−active and infected Aedes species mosquito such as Ae. aegypti and Ae. albopictus. Zika-virus infection poses a greater risk to humans as it can be transmitted from infected mother to fetus during pregnancy and cause birth defects in the new born. It can also be transmitted through sex, blood transfusion and laboratory exposure [3]. According to Centers for Disease Control and Prevention (CDC), a total of 8,580 Zika cases have been reported in the United States and its territories during the period of January 2015 to August 2016 (http://www.cdc.gov/zika/transmission/index.html). The mosquito vectors transmitting Zika-virus infection have also been widely reported to transmit other human diseases such as chikungunya and dengue. Zika-virus infection in human has been associated with the mild clinical symptoms and currently there is no vaccine available for its effective treatment [1]. Mosquito control methods have now arisen as promising alternative strategies to reduce the risk of human diseases transmitted through mosquito vectors [4,5].
Several strategies have been designed and implemented for controlling the vector mosquitoes. Synthetic insecticides have been effectively used during the past several decades against mosquito populations to eradicate the vector borne diseases [6]. The use of chemical insecticides has led to residual toxicity, biomagnifications and harmful effects on beneficial insects. Therefore, an increasing interest has been evinced in recent years for the development and application of bio-pesticides as vector control agents [7,8]. A large number of biocontrol agents were screened effectively for their efficacy, mammalian toxicity and environmental impact. The potential agents that have been investigated in mosquito control methods include viruses, fungi, bacteria, nematodes, protozoans and fishes. However, most of these biocontrol agents lack effective operational use due to difficulty in multiplying these agents in large quantities for application. However, the discovery of bacteria and their associated toxins to effectively kill different stages of mosquito species have opened up the possibility to use them as bio-pesticides in mosquito vector management program [9]. Besides these, additional interests have arisen to identify an effective and potential substance to trap a large number of oviposition females to the selective breeding sites for ease of control operation. This could reduce insecticidal consumption and will be cost effective [10,11].
The use of bacterial agents is probably the safest way in insect control as because it has no harmful chemicals, does not interact with mammals, fish or plants and is targeted directly at few insect species. Many commercial formulations using entomopathogenic bacterial strains were used as the biological control agents against vector mosquitoes [12,13]. The bacilli based mosquito larvicides have been developed and their control effect on several species of mosquito has been successfully demonstrated [14]. The toxins from Bacillus sphaericus (Bsp) and Bacillus thuringiensis subsp. israelensis (Bti) have been widely used in mosquito control programs [15,16]. Though, bacilli based toxins such as Cry and Cyt toxins for Bti and Bin toxins for Bspare powerful tools to control mosquito vectors, however, the development of resistance in different mosquito species have impeded the progress in using bacilli based biocides for mosquito control operation [9,17].
In recent decades, exotoxin from Pseudomonas fluorescens were reported to exhibit higher larvicidal and pupicidal activities against different mosquito species such Anopheles stephensi, Aedes aegptyi, Aedes albopictus, Culex quinquefasciatus, Armigeres subalbatus and Culex tritaenioahyncus [18-20]. Besides these bio-activities, the culture filtrate of Pseudomonas fluorescence also has efficient oviposition attractant activity against mosquito vectors, Ae. aegytpi and Ae. Albopictus [21]. Thus, exploring the multiple control effects of P. fluorescence such as larvicidal, pupicidal and oviposition attractant activities against the mosquito vectors, Ae. aegypti and Ae. albopictuscan provide new effective pathway for constructing lethal ovitrap to trap and kill these vectors in an efficient manner.
The strategic design involves the construction of ovitrap using culture supernatant of P. fluorescence to attract and trap large number of ovipositing females of Ae. aegypti and Ae. albopictus to the selective breeding sites for egg laying followed by knocking down of the larvae and pupae using exotoxins of P. fluorescence (Figure 1). For ovitrap construction, inoculate a loop full of P. fluorescence from an agar slope into 10 ml of nutrient broth and grow for 8h on a shaker at 250 rpm; then transfer the culture into fresh 250 ml of nutrient broth and incubate for 10 h. From this, transfer 5 ml of culture to 500 ml of production medium in 1 Liter flask and grow for 48 h. Then, harvest the cell mass by centrifugation at 10,000 rpm for 10 min and use the cell-free culture supernatant directly as a ovitrap to attract and trap ovipositing females of Zika vectors [21]. On other hand, grow P. fluorescence in glucose peptone salt (GPS) medium for 48 h and then extract exotoxin from the culture supernatant by (50%) ammonium sulfate precipitation [18]. Perform bioassay to determine the concentration of exotoxin sufficient to cause larval and pupal mortality. Further, incorporate desired concentration of exotoxin into constructed ovitrap that will efficiently knock down the larvae and pupae of trapped mosquitoes at the breeding sites.
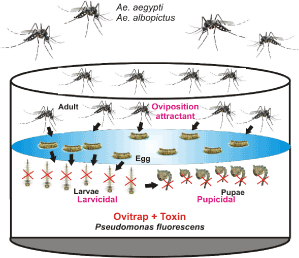
Figure 1. Strategy to trap and control Zika-virus carrying mosquitoes-Construction of ovitrap using culture supernatant of P. fluorescenceto attract and trap large number of ovipositing females of Ae. aegypti and Ae. albopictus to the selective breeding sites for egg laying followed by knocking down of the larvae and pupae using exotoxins of P. fluorescence.
This strategy can aid in construction of lethal ovitrap device to attract gravid female container-breeding mosquitoes (i.e Aedes aegypti, Aedes albopictus) and kill them. By adding lethal substances (exotoxin) to the ovitrap halts the life cycle of mosquito vectors by killing larvae and pupae at the breeding sites. This approach can be applied to monitor the distribution and density of Zika vectors by collecting eggs, which could be counted or hatched to identify specific types of mosquito. The use exotoxin from natural isolates of P. fluorescensi in ovitrapis also safe, reduce the insecticidal consumption and cost effective (~ 3 to 5$) with efficient operational use of this strategy in Zika outbreak and disease prevention.
- Fauci AS, Morens DM (2016) Zika Virus in the Americas-Yet Another Arbovirus Threat. N Engl J Med 374: 601-604. [Crossref]
- Yakob L, Walker T (2016) Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health 4: e148-149. [Crossref]
- Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, et al. (2016). Zika virus: medical counter measure development challenges. PLoS Negl Trop Dis 10: e0004530.
- Gould EA, Gallian P, De Lamballerie X, Charrel RN (2010) First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality! CMI 16: 1702-1704.
- Goddard J (2016) Essential Facts About Mosquito Control and Zika Virus. Am J Med 129: 1149-1152. [Crossref]
- Shaalan EAS, Canyon D, Younes MWF, Abdel-Wahab H, Mansour A H (2005) A review of botanical phytochemicals with mosquitocidal potential. Environ Int 31: 1149-1166.
- Ural MS, Saglam N (2005) A study on the acute toxicity of pyrethroid deltamethrin on the fry rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Pestbp 83: 124-131.
- Paine MJ, Brooke B (2016) Insecticide Resistance and Its Impact on Vector Control. In Advances in Insect Control and Resistance Management pp: 287-312.
- Poopathi S, Tyag BK (2004) Mosquitocidal toxins of spore forming bacteria: recent advancement. African Journal of Biotechnology 3: 643-650.
- Sivgnaname N, Amalraj DD, Kalyanasundaram M, Das PK (2001) Oviposition attractancy of an infusion from a wood inhabiting fungus for vector mosquitoes. Indian Journal of Medical Research 114: 18.
- Tyagi BK (2016) Advances in Vector Mosquito Control Technologies, with Particular Reference to Herbal Products. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization pp: 1-9.
- Nielsen-Leroux C, Charles JF, Thiery I, Georghiou GP (1995) Priority Paper Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border. European Journal of Biochemistry 228: 206.
- Paulraj MG, Kumar PS, Ignacimuthu S, Sukumaran D (2016) Natural Insecticides from Actinomycetes and Other Microbes for Vector Mosquito Control. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization pp: 85-99.
- El-Bendary M1, Priest FG, Charles JF, Mitchell WJ (2005) Crystal protein synthesis is dependent on early sporulation gene expression in Bacillus sphaericus. FEMS Microbiol Lett 252: 51-56. [Crossref]
- Cetin H, Oz E, Yanikoglu A, Cilek JE (2015). Operational Evaluation of Vectomax® WSP (Bacillus thuringiensis Subsp. israelensis+ Bacillus sphaericus) Against Larval Culex pipiens in Septic Tanks 1. Journal of the American Mosquito Control Association 31: 193-195.
- Zhang Q, Hua G, Adang MJ (2016) Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. [Crossref]
- Dovrat D, Aharoni A (2016) Bioengineering: Evolved to overcome Bt-toxin resistance. Nature 533: 39-40. [Crossref]
- Pushpanathan M, Pandian RS (2008) Management of dengue and chikungunya vectors Aedes aegypti (Linn) and Aedes albopictus (Skuse) (Diptera: Culicidae) by the exotoxin of Pseudomonas fluorescens Migula (Pseudomonadales: Pseudomonadaceae). Current Biotica 2: 74-103.
- Rajan VV, Pandian RS (2008) Larvicidal and pupicidal activities of the exotoxin of Pseudomonas fluorescens (Migula) isolates against the brain fever vectors, Armigeres subalbatus Coquillett and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Current Biotica 2: 32-40.
- Preethi SV, Pandian RS (2009) Evaluation of the larvicidal and pupicidal activities of the exotoxin of Pseudomonas fluorescens Migula against Aedes aegypti (L.) and Culex quinquefasciatus Say. Current Biotica 3: 416-438.
- Pushpanathan M, Pandian RS (2008) Culture filtrate of Pseudomonas fluorescens Migula an ideal ovitrap agent for the vectors of dengue and chikungunya, Aedes aegypti (Linn.) and Aedes albopictus (Skuse). Current Biotica 2: 461-471.
2021 Copyright OAT. All rights reserv

