Abstract
Plasmablastic lymphoma (PBL), a very aggressive variant of diffuse large B cell lymphoma (DLBCL), has been described initially as a rapidly progressive and almost invariably fatal diffuse large-cell lymphoma with plasmablastic features. Immunohistochemical findings showed generally CD20 antigen negative and VS38c, CD138 and MUM 1 positives. This neoplasm was initially described almost exclusively involving the jaw and oral mucosa in HIV-positive patients. However, most recently, the clinical spectrum of the disease has been expanding, with a number of single case reports in HIV-negative patients and with extra-oral manifestations.
Keywords
plasmablastic lymphoma; abdominal mass; AIDS; HIV
Introduction
AIDS-related lymphomas include a wide spectrum of aggressive and heterogeneous tumors that can be classified in four types: diffuse large B cell lymphomas (DLBCL), Burkitt lymphoma (BL); primary effusion lymphoma (PEL) and plasmablastic lymphoma (PBL) [1]. According to the World Health Organization (WHO), PBL is classified as a variant of DLBCL accounting for 2,6% of Non-Hodgkin lymphomas (NHL) in the setting of HIV infection [2].
Initially, PBL was described arising generally the soft tissue of the oral cavity and the jaw in HIV serological population [3]. However, extraoral sites can be involved as primary location of the neoplasia or concurrent of the oral lesions. The characteristic immunophenotype of PBL includes the presence of plasmablastic cells markers. In the last years, PBL has been diagnosed in HIV negative patients with other immunodeficiency such as transplant recipient or elderly patients [4].
Herein, we present two cases of PBL with large abdominal masses as primary manifestation of neoplastic disease.
Case 1
A 51-year-old man, infected with the human immunodeficiency virus (HIV) was admitted to our Department of HIV/AIDS related illness with two weeks history of vomiting, progressive abdominal pain predominantly on the periumbilical region and in the left flank, night sweats and weight loss (8 kg). He was receiving highly active antiretroviral therapy (HAART) based on abacavir, lamivudine and atazanavir boosted with ritonavir with a good immunological and virological response. At this moment, the CD4-T-cell count was 833 cells/µL and the plasma viral load was undetectable (<40 copies/mL). In the physical inspection collateral circulation of the abdominal wall was evidence. Relevant physical examination showed a large and painful abdominal mass which occupied the epigastrium, periumbilical region and both the right and left flank. Relevant laboratory findings included: haematocrit 36%, haemoglobin 13 g%, white blood cell count 10.8 x 103/L, platelets 257 x 106, erythrocyte sedimentation rate 63 mm/1st h and lactate dehydrogenase (LDH) 1941 U/L. Renal and liver function were normal.
Abdominal ultrasound showed a large and heterogeneous mass surrounding arterial vessels and displaces the pancreas and left renal flank that measures 17 cm x14 cm (Figure 1). Liver was normal and the spleen wasn’t seen because he had history of splenectomy. The rest of the peritoneum was no assessable because of the interposition of bowel loops. A computed tomography (CT) scan of the abdominal cavity revealed an extensive solid formation that hint the prevertebral retroperitoneum and progress to the umbilical region with a transverse diameter of 17 cm. A lymph node conglomerate extended in both internal and external iliac chains was seen.
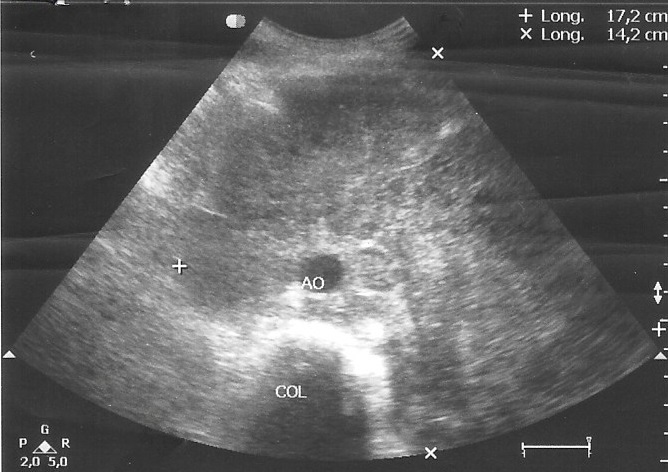
Figure 1. Abdominal ultrasound corresponding to patient 1, showed a large and heterogeneous mass surrounding arterial vessels and displaces the pancreas and left renal flank.
An exploratory laparotomy was done and a partial surgical resection of the abdominal mass was made. The samples were initially fixed in formalin, processed routinely and embedded in paraffin. It was stained with hematoxylin and eosin (H/E). Histopathological examination of the biopsy smears using H/E showed a diffuse and dense proliferation of large atypical lymphoid cells with areas of necrosis with plasmablastic differentiation with eccentric big round to oval nuclei, dense or dispersed chromatin, prominent nucleoli and abundant amphophilic cytoplasm with increased apoptosis and mitosis and scattered inflammatory cells (Figure 2). Immunohistochemistry (IHC) was performed; the antigen retrieval with low and high pH solution (VECTOR) following the protocol. The markers were CD20 (Clone L26) (DAKO); CD3 Monoclonal Mouse Anti-Human) (DAKO); MUM1 (Clone MUM1p) (DAKO) and Ki 67(Clone MIB-1) (DAKO). The detection methods include LSAB+ with diaminobencidine (DAKO). The percentage of the cells with Ki67 was counted ten fields per high-power field (HPF). In situ hybridization (ISH) for Epstein-Barr Virus genome (EBERs) was also performed. PNA Probe/Fluorescein (DAKO) was performed. IHC examination revealed that neoplastic cells were strongly positive for the plasma cells markers CD79a and MUM1 (Figure 3), being negative for CD138, CD45, CD3 and CD20 expression. The Ki67 index was high (90% expression). Based on the histopathological morphology and the plasma cell immunophenotype, the final diagnosis of high grade aggressive nodal primary PBL was made. ISH revealed extensive positive for EBERs (Figure 4). Bone marrow biopsy was negative for neoplastic infiltration.
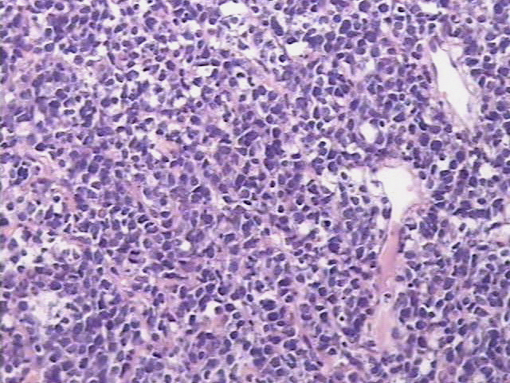
Figure 2. H/E showed a diffuse and dense proliferation of large atypical lymphoid cells with plasmablastic differentiation (patient 1).
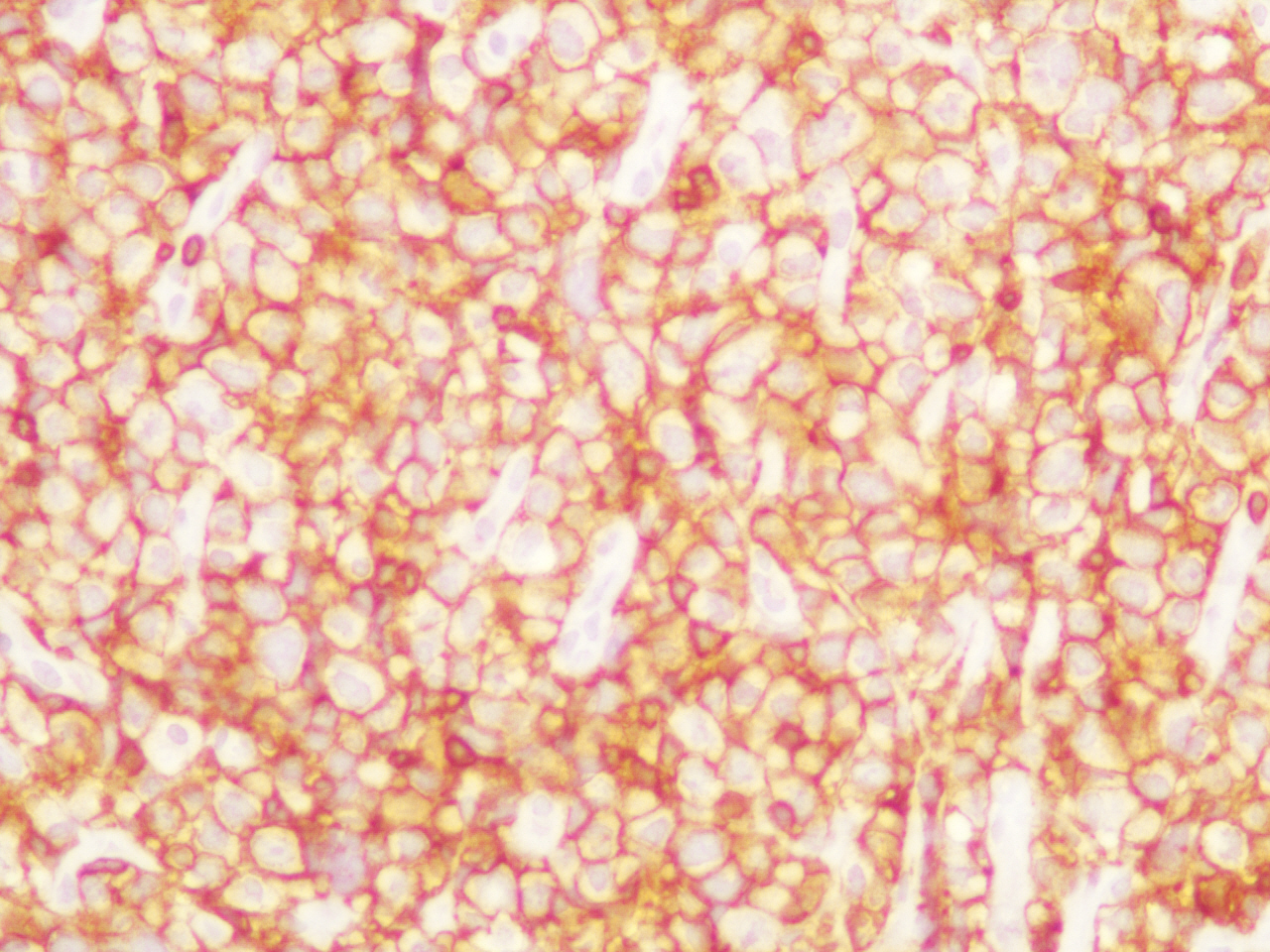
Figure 3. IHC examination revealed that neoplastic cells were strongly positive for the plasma cells markers MUM1 (patient 1).
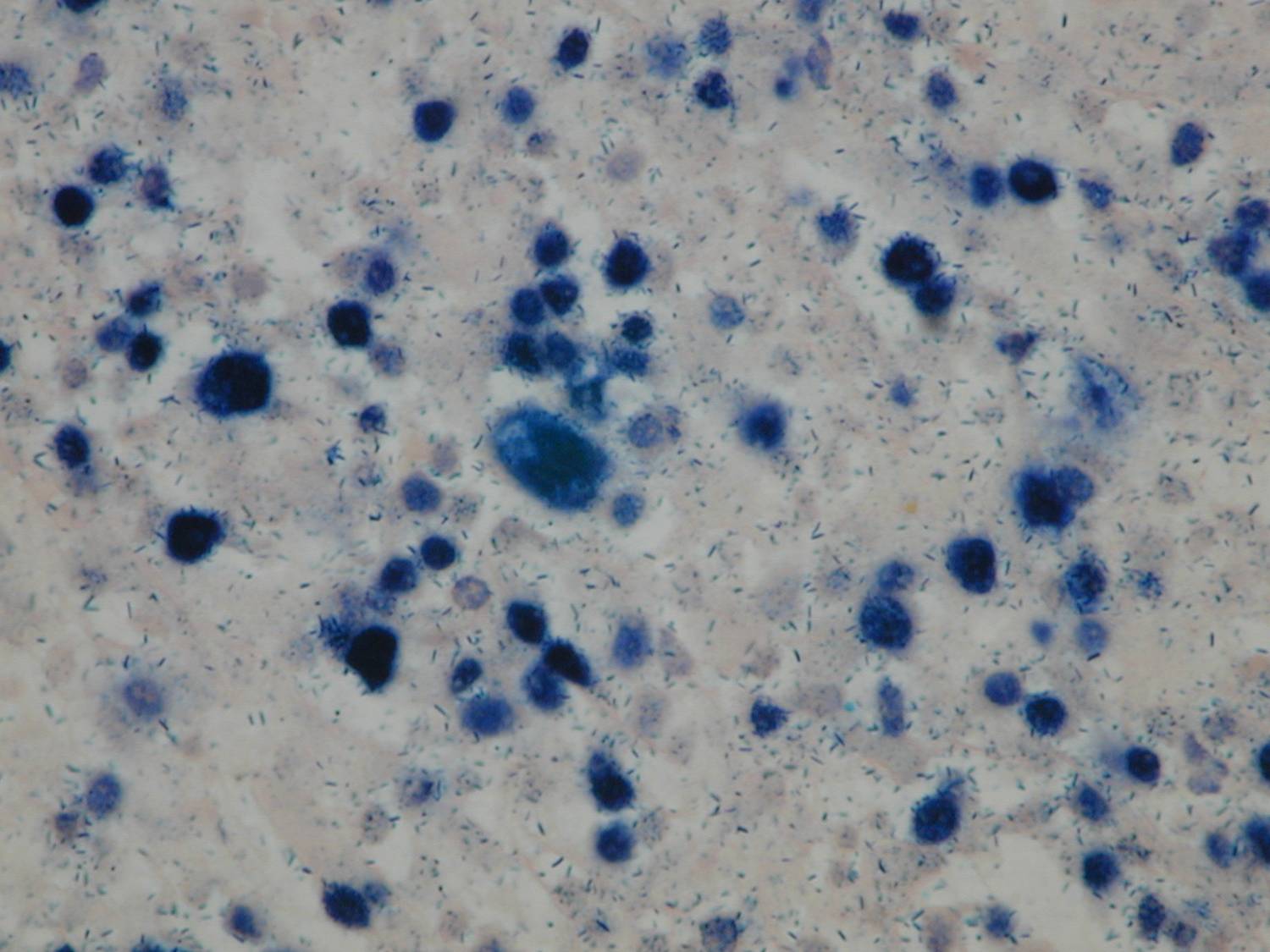
Figure 4. ISH revealed extensive positive for EBERs (patient 1).
Case 2
A 36-year-old man, infected with the human immunodeficiency virus (HIV) and with diagnosis of AIDS because he has history of intestinal microsporidiosis, cryptosporidiosis and AIDS associated colangiopathy. He was admitted to our Department of HIV/AIDS related illness with one month history of progressive abdominal pain predominantly on the periumbilical region and in the left flank, night sweats and weight loss (8 kg). He was receiving highly active antiretroviral therapy (HAART) based on tenofovir, emtricitabine and raltegravir, with a good immunological and virological response. At this moment, the CD4-T-cell count was 290 cells/µL and the plasma viral load was undetectable (<40 copies/mL). Relevant physical examination revealed a painful abdominal distension with a large and firm palpable abdominal mass which occupied the periumbilical region and the left flank. Also a firm and mobile non-tender neck mass was noted in the left supraclavicular fossa.
Relevant laboratory findings included: haematocrit 31%, haemoglobin 9,3 g%, white blood cell count 6.6 x 103/L, platelets 663 x 106, erythrocyte sedimentation rate 8 mm/1st h and lactate dehydrogenase (LDH) 276 U/L. Renal and liver function were normal.
Abdominal ultrasound showed multiple and enlarged mesentery and retroperitoneal hypoechoic adenopathies with ascites and minimal pleural effusion. Liver and pancreas were normal and a homogeneous splenomegaly was also observed (144 mm). A CT scan of the abdominal cavity revealed a large and heterogeneous retroperitoneal lymph node conglomerate which occupied the prevertebral region, the root of the mesentery, the intercavo aortic space and it extended to the left iliac fossa. CT scan of the neck and thorax revealed only the lymph nodal package on the left supraclavicular fossa.
An incisional and complete biopsy of the left supraclavicular adenopathy was performed.
The samples were initially fixed in formalin, processed routinely and embedded in paraffin. It was stained with hematoxylin and eosin (H/E). Histopathological examination of the biopsy smears using H/E showed a diffuse and dense proliferation of large atypical lymphoid cells with areas of necrosis. IHC was performed with similar method used in case 1. IHC examination revealed that neoplastic cells were strongly positive for the plasma cells markers MUM1, VS38c, CD138 with partial expression of CD45, CD79a and CD10. All other negative markers include CD3, CD20, BCL2 and BCL6. The Ki67 index was high (95-98% expression). Based on the histopathological morphology and the plasma cell immunophenotype, the final diagnosis of high grade aggressive nodal primary PBL was made. ISH revealed extensive positive for EBERs. Bone marrow biopsy was negative for neoplastic infiltration.
Discussion
HIV infection and AIDS are associated to increased risk for the development of lymphoproliferative disorders. Plasmablastic lymphoma (PBL) is a rare and aggressive B-cell lymphoma that was initially described almost exclusively in human immunodeficiency virus (HIV) infected patients. According with the WHO classification for lymphoproliferative disorders, PBL is a distinct variant of DLBCL that was initially described by Delecluse el al. [1] in 1987 arising, generally, in the soft tissue of the oral cavity and the jaw in HIV-seropositive population. Although PBL presents generally in the oral cavity with local invasion and rapid dissemination to extraoral sites, it has been reported in other sites such as stomach, liver, cervical lymph nodes, lungs, orbit and paranasal sinuses [5-9]. More than 20% of extra-oral PBL involve the lymph nodes, as we could see in our patients [10]. Extraoral sites of PBL are rare but show a similar invasive and rapid disseminated capability as in the oral cavity location. The majority of cases of HIV-associated PBL affected young males, less than 40 years and frequently is characterized by advanced stage of disease at diagnosis, extranodal sites involvement, elevated lactate dehydrogenase (LDH) levels and high International Prognosis Index (IPI) scores [11]. Additionally, some patient presents with the typical “B” symptoms of lymphoma as prolonged fever, night sweats and weight loss [12]. B symptoms have been reported in 33% of HIV-positive and 50% of HIV-negative patients at diagnosis [11]. Bone marrow involvement and B symptoms occurred in almost 40% of HIV-positive individuals and are relatively less common (25%) in HIV-negative individuals. None of our patients showed bone marrow infiltration [13].
The histological profile of PBL includes the expression of plasma cells markers such as CD38, CD138 and the absence or weak expression of lymphocyte common antigen (LCA) and B-cells markers such as CD20 and CD45. Immunohistochemistry (IHC) shows that PBL have DLBCL morphology with no B-cell immunophenotype but with plasma cell immunophenotype. Pathologically, the most typical characteristic of PBL is that this neoplasm expresses plasma cell markers in all cases, such as VS38c, CD38, CD138 and MUM 1, similar to plasma myeloma [14]. Also, PBL can often express the B-cell marker CD79A, as we could see in the two patients. Lack of CD20 expression with presence of CD38/CD138 is neither typical nor characteristic for PBL, it is also seen in plasma cell neoplasms, ALK+ large B-cell lymphoma and it can be seen in primary effusion lymphoma. Ki67 antigen identify by IHC is generally high in PBL, > 80%, in the biopsy performed on paraffin-embedded sections [4]. Our 2 patients had a Ki67 antigen > of 90%.
EBV-encoded RNA (EBER) Epstein-Barr virus (EBV) can be associated with the pathogenesis of PBL. EBV-encoded RNA (EBERs) is detected by in situ hybridization in about 70% of cases of PBL [15]. In a recent study, EBV infection based on EBERs expression was more commonly seen in HIV-positive patients (75%) and in patients with PBL arising in the post-transplant setting (67%) than in immunocompetent patients (50%) [13]. Most recently, an association between PBL and human herpesvirus 8 (HHV-8) has been described, but the pathogenic role of HHV-8 is unclear in comparison with the EBV [16].
The prognosis of PBL in the setting of HIV has also been historically poor. PBL in AIDS patients is generally associated with a poor prognosis due to the nature relapsing evolution of this neoplasm and the severe immunosuppression associated with the advanced HIV/AIDS disease. The most frequent cause of death in these patients is the lymphoma progression [17]. However, the prognosis of HIV-associated PBL can be improved with the addition of highly active antiretroviral therapy (HAART) to chemotherapy [18].
Treatment of PBL in HIV/AIDS patients is similarity to other aggressive lymphomas in this kind of patients including HAART plus chemotherapy. Patients with PBL who do not received chemotherapy died with a median survival of 3 months [11,19]. CHOP (cyclophosphamide, vincristine, doxorubicin and prednisone) or CHOP-like regimens are the most common therapeutics alternatives. In patients with PBL associated with AIDS the best therapeutic approaches include the combination of chemotherapy plus HAART. Therapy based on HAART plus CHOP was associated with improved survival in this kind of patients [11,20]. Also, some studies evaluated the use of other regimens more intensive than CHOP, such as infusional etoposide, doxorubicin, vincristine, cyclophosphamide and prednisone (EPOCH), hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (Hyper-CVAD), bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide and etoposide (VDT-PACE) or CODOX-M/IVAC [18,21-26].
Castillo et al. [11,20] analyzed retrospectively 70 patients with HIV-associated PBL treated with chemotherapy. In this series, the mean of CD4 + T cell count was 165 cells/µL, the overall response rate to chemotherapy was 77% but only 46% of the patients presented complete response. The overall survival rate was 14 months and 72% of patients died for lymphoma progression. In this study, the use of regimens more intensive than CHOP did not associated with a better survival. Also, a few number of cases has been published with spontaneous remissions of PBL in AIDS patients treated with HAART alone [27,28].
The large clinical spectrum of PBL in AIDS patients includes the possibility that this neoplasm can appear as a manifestation of immune reconstitution inflammatory syndrome (IRIS) or represents the first sign of a undiagnosed infection by HIV [29,30]. Finally, due to the strongly association between PBL and HIV infection, all patients with diagnosis of PBL should be tested for HIV infection.
Authorship
All authors mentioned above gave substantial intellectual contribution to this manuscript.
Funding Information
The authors received no specific funding for this work.
Competing InterestThe authors declare that they have no competing interests.
References
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, et al. (1997) Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 89: 1413-1420. [crossref]
- Gloghini A, Dolcetti R, Carbone A (2013) Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol 23: 457-467. [crossref]
- Corti M, Villafañe MF, Bistmans A, Campitelli A, Narbaitz M, et al. (2011) Oral cavity and extra-oral plasmablastic lymphomas in AIDS patients: report of five cases and review of the literature. Int J STD AIDS 22: 759-763. [crossref]
- Hansra D, Montague N, Stefanovic A, Akunyili I, Harzand A, et al. (2010) Oral and extraoral plasmablastic lymphoma: similarities and differences in clinicopathologic characteristics. Am J Clin Pathol 134: 710-719. [crossref]
- Metta H, Corti M, Maranzana A, Schtirbu R, Narbaitz M, et al. (2009) Unusual case of plasmablastic non-Hodgkin's lymphoma located in the liver. First case reported in an AIDS patient. Ann Hepatol 8: 242-245. [crossref]
- Avilés-Salas A, Herrera-Goepfert R, Aguilar-León D, Candelaria-Hernández M, Martínez-Cordero E, et al. (2011) Plasmablastic lymphoma of the gastrointestinal tract in AIDS patients. Medicina (Buenos Aires) 71: 536-541.
- Corti M, Villafañe Fioti MF, Lewi D, Schtirbu R, Narbaitz M, et al. (2006) [Non-Hodgkin's lymphomas of the digestive tract and anexal glands in AIDS patients]. Acta Gastroenterol Latinoam 36: 190-196. [crossref]
- Corti M, Villafañe MF, Bistmans A, Campitelli A, Narbaitz M (2013) Soft-tissue masses as presentation of non-Hodgkin's lymphoma in AIDS patients. An Bras Dermatol 88: 631-634. [crossref]
- Tavora F, Gonzalez-Cuyar LF, Sun CC, Burke A, Zhao XF (2006) Extra-oral plasmablastic lymphoma: report of a case and review of literature. Hum Pathol 37: 1233-1236. [crossref]
- Sarode SC, Zarkar GA, Desai RS, Sabane VS, Kulkarni MA (2009) Plasmablastic lymphoma of the oral cavity in an HIV-positive patient: a case report and review of literature. Int J Oral Maxillofac Surg 38: 993-999. [crossref]
- Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, et al. (2010) Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma. Oncologist 15: 293-299. [crossref]
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, Redfield RR, Gilliam BL (2008) Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis 8: 261-267. [crossref]
- Morscio J, Dierickx D, Nijs J, Verhoef G, Bittoun E, et al. (2014) Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and post-transplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol 38: 875-886.
- Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, et al. (2005) Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol 18: 806-815. [crossref]
- Cesarman E (2011) Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 305: 163-174. [crossref]
- Carbone A, Gloghini A, Gaidano G (2004) Is plasmablastic lymphoma of the oral cavity an HHV-8-associated disease? Am J Surg Pathol 28: 1251-1252. [crossref]
- Panos G, Karveli EA, Nikolatou O, Falagas ME (2007) Prolonged survival of an HIV-infected patient with plasmablastic lymphoma of the oral cavity. Am J Hematol 82: 761-765. [crossref]
- Lester R, Li C, Phillips P, Shenkier TN, Gascoyne RD, et al. (2004) Improved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of highly active antiretroviral therapy: a report of two cases. Leuk Lymphoma 45: 1881-1885. [crossref]
- Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, et al. (2010) Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma 51: 2047-2053. [crossref]
- Castillo JJ, Reagan JL (2011) Plasmablastic lymphoma: a systematic review. ScientificWorldJournal 11: 687-696. [crossref]
- Teruya-Feldstein J, Chiao E, Filippa DA, Lin O, Comenzo R, et al. (2004) CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol 15: 1673-1679. [crossref]
- Tirelli U, Spina M, Gaidano G, Vaccher E, Franceschi S, et al. (2000) Epidemiological, biological and clinical features of HIV-related lymphomas in the era of highly active antiretroviral therapy. AIDS 14: 1675-1688. [crossref]
- Bohlius J, Schmidlin K, Costagliola D, Fätkenheuer G, et al. (2009) Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group, Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS 23: 2029-2037. [crossref]
- Mounier N, Spina M, Gabarre J, Raphael M, Rizzardini G, et al. (2006) AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood 107: 3832-3840. [crossref]
- Simcock M, Blasko M, Karrer U, Bertisch B, Pless M, et al. (2007) Treatment and prognosis of AIDS-related lymphoma in the era of highly active antiretroviral therapy: findings from the Swiss HIV Cohort Study. Antivir Ther 12: 931-939. [crossref]
- Navarro JT, Ribera JM, Oriol A, Tural C, Millá F, et al. (2003) Improved outcome of AIDS-related lymphoma in patients with virologic response to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 32: 347-348. [crossref]
- Armstrong R, Bradrick J, Liu YC (2007) Spontaneous regression of an HIV-associated plasmablastic lymphoma in the oral cavity: a case report. J Oral Maxillofac Surg 65: 1361-1364. [crossref]
- Corti M, De Carolis L, Campitelli A, Narbaitz M, Baré P (2012) Response of human immunodeficiency virus-associated oral plasmablastic lymphoma to highly active antiretroviral therapy without chemotherapy: case report and literature review. Infect Dis Clin Pract 20: 79-81.
- Corti M, Solari R, Cangelosi D, De Carolis L, Schtirbu R, et al. (2007) Oral cavity lymphoma as secondary AIDS-defining neoplasm in a patient on HAART with immune reconstitution. Rev Soc Bras Med Trop 40: 582-584. [crossref]
- Powles T, Thirlwell C, Nelson M, Bower M (2003) Immune reconstitution inflammatory syndrome mimicking relapse of AIDS related lymphoma in patients with HIV 1 infection. Leuk Lymphoma 44: 1417-1419. [crossref]




