Components of the glycocalyx are being intensively studied for their involvement in a vast array of glomerular diseases. Among the most abundant proteoglycans in the glomerular cell glycocalyx are the heparan sulfate proteoglycans Glypican-1 and 5, expressed predominantly in glomerular endothelial and epithelial cells, respectively.
We used a previously characterized zebrafish model to define the role of all different in zebrafish existing isoforms of Glypican-1 (a and b) and 5 (a,b and c) in vascular and renal pathology and tested the hypothesis that Glypican expression is important for the integrity of the glomerular barrier. Glypican morpholino knockdown in l-fabp:DBP-eGFP fish showed edematous phenotypes and a significant reduction of eye fluorescence for Glypican-1a, 5a und 5c, but not for Glypican-1b and 5b isoforms. This indicates a significant loss of plasma proteins following knockdown of Glypican-1a, 5a and 5c in contrast to a conserved filtration barrier following knockdown of Glypican-1b and 5b isoforms. Knockdown of all different examined Glypican isoforms did not interfere with early nephron development as shown in the WT1b fish strain. Using electron microscopy, we could demonstrate that Glypican-1aknockdown leads to a predominantly endothelial cell injury with preservation of podocyte foot processes, while knockdown of Glypicans- 5a and 5c shows focal podocyte effacement with conserved endothelial structures. Ultrastructural analysis of glomeruli following knockdown of Glypicans-1b and 5b reveals normal glomerular structure.
In this brief study we demonstrate in an in-vivo model that the Glypican proteoglycan family is critical in vascular and glomerular integrity. Our observations suggest a role for Glypican- 1a in endothelial cell and Glypican-5a and 5b in podocyte cell injury leading to subsequent loss of glomerular filtration barrier function and proteinuria.
Abbreviations: hpf: hours post fertilization
Quantitative changes in heparan sulfate proteoglycans have been associated with a wide range of inflammatory and proteinuric nephropathies, including diabetic nephropathy, minimal change disease, membranous nephropathy, systemic lupus associated nephropathy and various rodent models of nephrotic syndrome [1,2]. Of the diverse Glypican proteoglycan family Glypicans-1, 3, 4 and 5 are found in the human kidney. [3-5]. Recently, Okamoto et. al. identified through a genome wide association study and replication analysis a certain variant of Glypican-5 as a new susceptibility gene for acquired nephrotic syndrome [6]. Glypican-1 is one of the most important cell surface associated glomerular heparan sulfate proteoglycans and is expressed in both glomerular epithelial as well as glomerular endothelial cells 1. However, not much is known until now about a possible association of Glypican-1 with kidney disease. While in humans only six different Gypican proteins exist [7], in zebrafish ten different Glypican proteins have been isolated [8]. In contrast to humans, in zebrafish two different isoforms of Glypican-1, named Glypican-1a and 1b, and three different isoforms of Glypican 5, named Glypican-5a, 5b and 5c, have been identified 8.
In this present study we examined the role of the Glypican-1 (a and b) and Glypican-5 (a,b and c) genes in glomerular and vascular physiology employing a zebrafish model. We present evidence for an important role of this protein in the development of proteinuria as well as vascular permeability and describe for the first time a model of glomerular pathology of Glypican-1 and Glypican-5 in an in-vivo setting.
All methods applied in this study have been extensively described by our laboratory previously 9, and will therefore be described in a short fashion.
Zebrafish stocks and embryos
Zebrafish, Wildtype AB and the Transgenic zebrafish lines Tg(l-fabp:DBP-eGFP) 10, Tg(flk:mcherry/l-fabp:DBP-eGFP) and Wt1b-GFP 11, were grown and mated at 28.5°C. Embryos were kept and handled in standard E3 solution buffered with 2 mM HEPES as previously described previous 12,13. All zebrafish studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) under approval #14-06.
Zebrafish morpholino injection
As decribed before by our group, Glypican-1a and 1b, Glypican 5a, 5b and 5c as well as control morpholinos were injected into one to four-cell stage fertilized embryos at a concentration of 100µM. Morpholino sequences were ordered from GeneTools (Philomath, OR) as follows: control sequence - 5’- CCTCTTACCTCAGTTACAATTTATA-3’, Glypican-1a sequence 5’- AAAAAGAGCTGTTGACTCACTGTCA-3’, Glypican-1b sequence 5’- CAGCAGGACCGATTCACCTACCGGA-3’, Glypican-5a sequence 5’- TAACCACACACTCATCCTCTCACCT-3’, Glypican-5b sequence 5’- AATGTTTTTGGACCTACCTGGTTGA-3’ and Glypican-5c sequence 5’- ACATGACGGCATTTCAGTGGAGAAA -3’. The Glypican-1a/1b/5a/5b morpholino constructs act as a splice donor, initiating wrong pre-mRNA splicing in the region between exon2-intron2/exon1-intron1/exon1-intron1/exon7-intron7, respectively. The Glypican-5c morpholino construct works as a translation blocker by binding close to the translational start site of the′complementary mRNA in the 5 -untranslated region.
Fluorescence proteinuria measurements –fabp eye assay
72 hours post fertilization (hpf) dechorionated Tg(l- fabp:DBP-eGFP) embryos were anesthetized with a 1:20 to 1:100 dilution of 4 mg/ml Tricaine, placed in 200 µl E3 in single wells of 96 well plates and allowed to recover. Sequential images of live fish were then generated 96 and 120hpf using the Axiovert 200 microscope connected to an AxioCam charge-coupled device camera, and images were taken using the Axio Vision release 4.5 SP1 software package. The maximum fluorescence intensities of grayscale images of the pupil of the fish were measured using NIH’s ImageJ application and reported in relative units of brightness as previously described by our group.
Assay for glomerular fusion – WT1b fish
Normal development and the fusion of the zebrafish pronephros was documented in the WT1b:eGFP fish line 72hpf for all Glypican isoforms at a morpholino concentration of 100 µM.
Histology and transmission electron microscopy (TEM)
Morphant larval zebrafish were sampled at 120 hpf and fixed , washed and embedded with epon according to manufacturer’s protocol (Hard Plus Resin 812, Electronmicroscopy Sciences, Hatfield, PA). Ultrathin sections of 70-90 nm were imaged using a JEOL-1230 or a Morgagni (FEI, Eindhoven) transmission electron microscope, operated at 80 kV.
Statistics
We used mean ± SEM throughout this study. For comparing two different groups of eye fluorescencé T results, Student test was applied. p-values (2-sided) were considered significant at p<0.05. Statistical analysis was performed using GraphPad Prism Software (San Diego, California).
Knockdown of Glypican-1a, 5a and 5c in zebrafish induces a renal phenotype
Following injection of 100 µM of Glypican-1a and b as well as Glypican 5a, b and c specific antisense morpholinos, we could detect the development of an edematous phenotype in Glypican-1a, 5a and 5c knockdown fish, but not in Glypican-1b and 5b knockdown fish. This phenotype was characterized by general body edema, including pericardial effusion, yolk sac edema and a severely arched back. Those fish displayed reduced activity and had high rates of mortality at 96 and 120 hpf. The phenotype was worst in Glypican-5c knockdown fish, and about equal severe in Glypican-1a and 5a knockdown fish. The heartbeat and blood flow of these fish was normal pointing towards normal cardiac development. Edema developed already at 72hpf and was progressing in severity by 96 and 120hpf. This phenotype was not seen in WT fish, fish injected with a control morpholino, and in fish injected with a Glypican-1b and 5b knockdown morpholino. Representative phenotype pictures are shown in Figure 1 A.
Knockdown of Glypican-1a, 5a and 5c leads to proteinuria
We measured the loss of high molecular weight GFP tagged DBP protein as loss of eye vasculature fluorescence employing the Tg(l- fabp:DBP-eGFP) fish line. This revealed a highly significant loss of fluorescence in the Glypican-1a, 5a and 5c knockdown fish compared with control fish for both 96hpf and 120hpf. Glypican-1b and 5b knockdown fish showed no significant loss of eye fluorescence, neither at earlier nor at later time points. Results are shown in Figure 1 B and where as follows: 96hpf: control = 48.1 ± 1.6, Glypican-1a = 23.5 ± 1.9, 1b = 43.2 ± 2.5, 5a = 23.2 ± 3.0, 5b = 47.7 ± 2.0, 5c = 13 ± 0.8 and 120hpf: control = 66.7 ± 3.2, Gypican-1a = 43.9 ± 7.1, 1b = 68.7 ± 5.3, 5a = 15.9 ± 4.6, 5b = 57.9 ± 3.9, 5c = 0.7 ± 0,1). Loss of fluorescence was most pronounced with knockdown of Glypican-5c, corresponding well with the highest severity of edematous phenotype seen with this knockdown. Knockdown of Glypican-1a and 5a were about equal at 96hpf and more pronounced with Glypican-5c at the later time point of 120hpf.
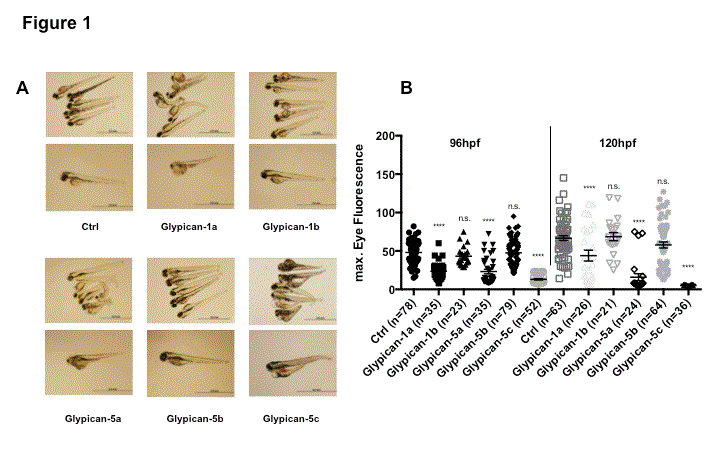
Figure 1.
Knockdown of Glypican-1a, Glypican-5a and Glypican-5c in zebrafish induces an edematous phenotype and proteinuria.
Edematous phenotype of Glypican knockdown (A). Compared are Glypican knockdown and control fish at 120hpf, morpholino concentration is 100µM. Glypican-1a, Glypican-5a and Glypican-5c knockdown fish exhibit yolk sac and pericardial edema with severely curved arches. In contrast, control fish, Glypican-1b and 5b knockdown fish retain their linear shape and show no edema.
Knockdown of Glypican-1a, Glypican-5a and Glypican-5c leads to proteinuria – Quantitative measurement of eye fluorescence (B). Compared are results for Glypican knockdown and control fish at a morpholino concentration of 100uM Eye fluorescence of Glypican-1a, Glypican-5a and Glypican-5c knockdown fish is highly significantly reduced compared to control as well as Glypican-1b and 5b knockdown fish at both 96 and 120hpf.
Values are means ± SEM. ns = non significant, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.001.
Knockdown of Glypicans does not interfere with early zebrafish nephron development.
The zebrafish embryonic pronephros consists of two nephrons with glomeruli fused at the embryo midline and two pronephric tubules that connect the glomerulus to the pronephric ducts, that fuse just before the cloaca [9,14 ]. As seen in Figure 2 we could demonstrate in the WT1b fish strain that knockdown of all different Glypican isoforms studied does not interfere with early nephron development. Two glomeruli give rise to two seperate tubular structures. Fusion of the two pronephros takes place about 72hpf.
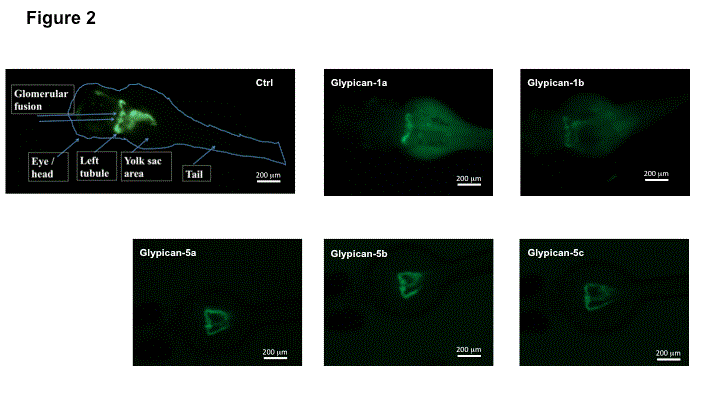
Figure 2.
Knockdown of Glypicans in zebrafish does not interfere with early zebrafish nephron development .
Fluorescence microscopy showing pronephron structures of WT, control morpholino and Glypican knockdown fish at 72hpf. In all fish two separate glomerular tufts and tubular structures and fusion of the two pronephrons are seen. There is no defect of development or disruption of fusion of the pronephrons in Glypican knockdown fish. Anatomical structures are labeled accordingly in the left picture.
Knockdown of Glypican-1a disrupts glomerular filter structure by causing endothelial cell injury, while knockdown of Glypican-5a and 5c leads to podocyte foot process effacement
To directly visualize the effect of Glypican knockdown on glomerular slit diaphragm architecture, we analyzed the glomeruli of 120hpf embryos by transmission electron microscopy. Diffuse endothelial cell swelling was seen in the glomeruli of Glypican- 1a knockdown fish, whereas the endothelium appeared healthy in control injected fish and wildtype fish as shown in Figure 3. However, regular podocyte foot processes and basement membrane structures were observed in Glypican-1a morpholino injected fish. When electron microscopy pictures of glomeruli following knockdown of Glypican-5a and 5c were analyzed, focal podocyte foot process effacement could be recognized in both isoforms. In contrast to Glypican-1a knockdown fish endothelial cell morphology and endothelial fenestrae appeared to be normal. For all Glypican isoforms a normal glomerular basement membrane structured could be recognized. In marked contrast, Glypican-1a and 5b knockdown fish showed normal ultrastructural glomerular anatomy comparable with control and wild type fish.
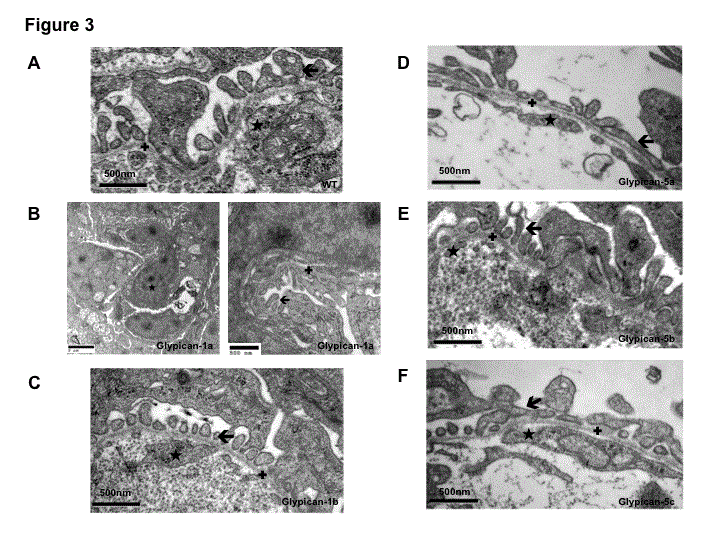
Figure 3.
Knockdown of Glypicans disrupts glomerular filter structure by causing endothelial cell and podocyte injury – Transmission electron microscopy analysis of glomeruli at 120hpf.
WT fish (A) as well as Glypican-1b (C) and 5b (E) knockdown fish show regular endothelial cell (★) and podocyte foot process morphology () as well as a normal basement membrane structure (✚).
Glypican-1a (B) knockdown fish glomeruli exhibit diffuse endothelial cell swelling (★), but regular podocyte foot processes () and basement membrane (✚) structures.14 In contrast, glomeruli of Glypican-5a (D) and 5c (F) knockdown fish show podocyte foot process effacement (), which has a focal character for both isoforms. Normal endothelial cell morphology (★) and glomerular basement membrane (✚) structures are seen in both isoforms.
In this manuscript, we present evidence for an important role of the Glypican protein family in the development of proteinuria as well as vascular permeability in a zebrafish in-vivo seeting. Evidence for this conclusion from the present experiments is as follows:
2021 Copyright OAT. All rights reserv
1). Glypican-1a, 5a and 5c knockdown fish develop a severely disturbed edematous phenotype that is not seen in control morpholino injected fish nor in Glypican-1b and 5b knockdown fish;
2). Employing a transgenic fish line expressing a GFP tagged DBP protein, Glypican-1a, 5a and 5c knockdown fish show a highly significant loss of eye fluorescence which corresponds to significant loss of high molecular weight protein via the urine, whereas Glypican-1b and 5b knockdown fish exhibit conserved vascular fluorescence
3). Proteinuria appears to correspond with the severity of the edematous phenotype, is worst in Glypican-5c knockdown fish and about equal in Glypican-1a and 5a knockdown fish
4). Renal pathology is not due to gross renal developmental defects as seen in the WT1b fish strain.
Proteinuria is considered to be a hallmark of glomerular pathology [15].
Given the fact that Glypican-1is expressed in both glomerular endothelial cells and in podocytes [3,16], we proceeded to investigate which cell type might be primary responsible for the disruption of glomerular filter integrity following Glypican knockdown in the zebrafish model. Our experiments provide further evidence that Glypican-1 gene knockdown leads to a predominant endothelial injury, while Glypican-5a and 5c results in a podocyte phenotype. Transmission Electron Microscopy pictures of Glypican-1aknockdown fish glomeruli showed substantial endothelial cell swelling whereas no significant podocyte effacement was seen and the basement membrane architecture principally remained intact. The observed tubular damage is most likely secondary to the predominant endothelial injury that is taking place in the glomerulus. In contrast, Glypican-5a and 5c knockdown fish showed focal podocyte effacement but conserved endothelial cell structures when glomerular structures were analyzed on an ultrastructural level using transmission electron microscopy. These electron microscopy findings provide direct evidence that the nephrotic phenotype observed in Glypican-1a knockdown fish is likely due to primary endothelial cell injury while Glypican-5a and 5c is associated with podocytic cell injury that both ultimately lead to disintegrity of the glomerular filter function and secondary tubular cell injury.
It is not clear why knockdown of Glypican-1a, 5a and 5c lead to glomerular injury, whereas Glypican-1b and 5b knockdown does not. Based on comparable expression patterns and partial synteny a previous investigation has concluded that Glypican-1a is more likely to be the true ortholog of mammalian Glypican-1 8. Of all three Glypican-5 isoforms, Glypican-5a has the highest amino acid sequence identity/similarity compared to mammalian Glypican-5, followed by Glypican-5c and then 5b.
In endothelial cells, Glypican-1 is also involved in flow sensation: Exposure to an increase in flow leads to mobilisation of Glypican-1 and caveolin 1 to the plasma membrane and the activation of endothelial nitric oxide synthase [17,18]. One could suspect, that Glypican-1 knockdown leads to structural disintegrity and maladaption of endothelial cell response to shear stress and thus to endothelial injury, glomerular filtration barrier dysfuntion and proteinuria. Among the different glypicans Glypican-1 is the only member expressed in the vascular system [19,20]. Both angiogenic and anti-angiogenic proteins, endostatin and VEGF-165, were shown previously to require interaction with Glypican-1 for the binding to their corresponding receptors in endothelial cells [16,21]. Glypican`s heparin sulfate proteoglycans are essential to chaperone both endostatin and VEGF binding. Endostatin appears to antagonize proangiogenic actions of VEGF not by cross-competition for receptor binding but rather via antagonizing intracellular signaling [21]. VEGF is able to cause endothelial dysfunction in patients with diabetic nephropathy and chronic kidney disease, whereas it appears to have a protective role in preeclampsia associated kidney disease [22]. It is conceivable that knockdown of Glypican-1 causes a dysbalance of VEGF and endostatin and therefore leads to endothelial dysfunction that we observe in the zebrafish model.
Recent studies focusing on the role of Glypican-5 in glomerular pathology, using cell culture experiments in rat glomerular epithelial cells and employing a podocyte specific Glypican-5 knockdown mouse model, have been suggested a close association of Glypican-5 with FGF2 signaling and podocyte injury 6. A potential harmful role of FGF2 signaling has been suspected in several different etiologies of nephrotic syndrome [23-25]. It is feasible, given the same podocyte phenotype we observed comparing Glypican-5a and 5c knockdown fish, that this is the predominant injury mechanism also for Glypican-5c knockdown associated podocyte injury.
In this present study we investigated in a zebrafish vivo model the role of the Glypican proteoglycan family in glomerular physiology and conclude that Glypican-1a, 5a and 5c play an important role in the integrity of the glomerular filtration barrier and reduced Glypican-1a/5a and 5c expression may contribute to injury to glomerular endothelial cells and podocytes, respectively, the loss of glomerular filtration barrier function and the onset of proteinuria.
Research reported in this publication was supported by Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM0103423 and P20GM104318. This work was also supported by SCHI587/3,4,6 granted to M.S. and N.H. was the recipient of an NIA (New Investigator Award) from MDIBL.
None declared.
- Rops AL, van der Vlag J, Lensen JF, Wijnhoven TJ, van den Heuvel LP, et al. (2004) Heparan sulfate proteoglycans in glomerular inflammation. Kidney Int 65: 768-785. [Crossref]
- Raats CJ, Van Den Born J, Berden JH (2000) Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int 57: 385-400. [Crossref]
- Litwack ED, Ivins JK, Kumbasar A, Paine-Saunders S, Stipp CS, et al. (1998) Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev Dyn 211: 72-87. [Crossref]
- Saunders S, Paine-Saunders S, Lander AD (1997) Expression of the cell surface proteoglycan glypican-5 is developmentally regulated in kidney, limb, and brain. Dev Biol 190: 78-93. [Crossref]
- Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND (2001) Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 231: 31-46. [Crossref]
- Okamoto K, Tokunaga K, Doi K, Fujita T, Suzuki H, et al. (2011) Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet 43: 459-463.
- Filmus J, Capurro M, Rast J (2008) Glypicans. Genome Biol 9: 224. [Crossref]
- Gupta M, Brand M. (2013) Identification and expression analysis of zebrafish glypicans during embryonic development. PLoS One 8: e80824. [Crossref]
- Hanke N, Staggs L, Schroder P, Litteral J, Fleig S, et al. (2013) "Zebrafishing" for novel genes relevant to the glomerular filtration barrier. Biomed Res Int 2013: 658270. [Crossref]
- Xie J, Farage E, Sugimoto M, Anand-Apte B (2010) A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol 10: 76. [Crossref]
- Bollig F, Mehringer R, Perner B, Hartung C, Schäfer M, et al. (2006) Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn 235: 554-561. [Crossref]
- Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, et al. (2007) Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746-1750. [Crossref]
- Kirsch T, Kaufeld J, Korstanje R, Hentschel DM, Staggs L, et al. (2013) Knockdown of thehypertension-associated gene NOSTRIN alters glomerular barrier function in zebrafish (Danio rerio). Hypertension 62: 726- 730. [Crossref]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, et al. (1998) Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655-1467. [Crossref]
- Tryggvason K, Patrakka J, Wartiovaara J (2006) Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387-1401. [Crossref]
- Gengrinovitch S, Berman B, David G, Witte L, Neufeld G, et al. (1999) Glypican-1 is a VEGF165 binding proteoglycan that acts as an extracellular chaperone for VEGF165. J Biol Chem 274: 10816-10822. [Crossref]
- Zeng Y, Tarbell JM2 (2014) The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One 9: e86249. [Crossref]
- Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, Tarbell JM (2014) Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol (Camb) 6: 338-347. [Crossref]
- Stein JM (1975) The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells. Biochem Pharmacol 24: 1659-1662. [Crossref]
- Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. (1997) Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest 100: S67-75. [Crossref]
- Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G. (1992) Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem 267: 20435-20443. [Crossref]
- Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, et al. (2001) Cell surface glypicans are low-affinity endostatin receptors. Mol Cell 7: 811-822. [Crossref]
- Doi K, Noiri E, Fujita T (2010) Role of vascular endothelial growth factor in kidney disease. Curr Vasc Pharmacol 8: 122-128. [Crossref]
- Ray PE, Liu XH, Xu L, Rakusan T. (1999) Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol 13: 586-593. [Crossref]
- Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, et al. (1995) Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest 96: 2809-2819.
- Kriz W, Hahnel B, Rosener S, Elger M. (1995) Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int 48: 1435-1450.
