Viscum album and its endophytic fungal species lectin are reported as antioxidant, antimicrobial, anti-inflammatory and anticancer activity. The present investigation was undertaken to in vitro (by enzymes inhibition) and in vivo antidiabetic activity of endopytic fungi, Alternaria species N-acetyl-galactosamine, 54 kDa protein lectin in alloxan induced diabetes in rats. Endophytic fungal lectinwas orally administered at 400 mg/kg body weight (BW) dose to alloxanized mice (blood glucose>200 mg/dl). Various diabetic parameters were studied and compared with untreated mice. Furthermore, Matrix Assisted Laser desorption Ionization Time Of Flight Mass Spectroscopy (MALDI-TOF-MS) was employed to reveal the characteristics and identification of peptides.The lectin was confirmed by hemagglutination, PAS staining and it is N-acetyl-galactosamine, 64 kDa glycoprotein. The endophytic fungal lectin inhibited the three important diabetic enzymes viz., α-amylase (85.26 ± 1.25),
α-glucosidase (93.41 ± 1.27) and sucrase (81.61 ± 1.05) strongly. The N-acetyl-galactosaminelectin treated diabetic rats showed significantly increased the body weight (8.50%) compared with the standard drug (9.01%) after 14 days of treatment. The lectin treated rats shown regeneration of pancreatic tissues, ducts and walls, acini, blood vessels, blood capillaries, islet capsules and cells and all these were in accordance with format only and recovery has been observed. The lectin treated diabetic rats also reduced the levels of urea (43.7 ± 5.8), creatinine (0.32 ± 0.01), serum cholesterol (103.54 ± 2.13), triglycerides (124.68 ± 2.49), aspertate aminotransferase (138) and alanine aminotransferase (57) and this results confirms the health of the animal when compared to standard drug. The study provides that endophytic fungal lectin could exert a protective effect against β-cell damage by its anti-inflammatory effects and aids regeneration might through stimulation of ductal stem cells. However, further experimental studies are still needed for more details on lectin as an adjuvant in the management of diabetes mellitus. The present results revealed that, endophytic fungal lectin possess potent anti-diabetic activity and regenerated the cells or tissues in diabetic rats.
endophytes, lectin, in vivo antidiabetic, histopathology, serum analysis
Plant endophytic fungi have been recognized as an important and novel resource of natural bioactive products with potential application in agriculture, medicine and food industry [1-3]. In the past two decades, many valuable bioactive compounds with antimicrobial, insecticidal, cytotoxic and anticancer activities have been successfully discovered from the endophytic fungi. These bioactive compounds could be classified as alkaloids, terpenoids, steroids, quinones, lignans, phenols and lactones [4,5].
A nonpeptidal fungal (Pseudomassaria sp.) metabolite acts as an insulin mimetic and unlike insulin, is not destroyed in the digestive tract and may be given orally and resulted in significant lowering of blood glucose levels [6]. Endophytic fungal extracts showed antidiabetic activity [7,8].
Plant lectins are proteins bind to specific carbohydrate groups on the plasma membranes of mammalian cells, inducing variety of biological effects [9]. Many reports are available on plant lectins antidiabetic activity by possessing various mechanisms [10-12].
The present day, the diabetes is aalmost become a serious public health problem, particularly in developed countries as a major threat to global development [13,14]. This shows the necessities and the importance of more alternate and effective antidiabetic drugs and their systematic studies to manage diabetes.
This investigation was aimed to evaluate endophytic fungal lectin in in vitro and in vivo on Alloxan induced rats and characterized the lectin. Hyperglycemia, hyperlipidemia and regeneration of histopathological structures were studied in lectin treated Alloxan induced diabetic rats. This is the attempt was made on endophyticlectin antidiabetic activity in in vivo.
Collection of endophytic fungi, Alternaria species and mass production
The endophytic fungi, Alternaria species was (previously isolated from leaf) collected from stock cultures of Department of Biotechnology, Shridevi Institute of Engineering & Technology, Sira Road, Tumakuru, Karnataka, India and was cultured CzapekDox broth for largescale cultivation, which was incubated at room temperature (26 ± 2°C) for 8 days.
Isolation and purification of lectin from endophytic fungi Alternaria species
Alternaria species lectin was obtained through a sequential purification protocol of Araujo et al. [15] and Rocha et al. [12]. The powdered fungal mycelial mat (10 g) was suspended in 0.15 M NaCl (100 mL). After homogenization in a magnetic stirrer (16h at 4°C), followed by filtration through gauze and centrifuged (4,000 xg, 15 min), the supernatant (crude extract) was taken as starting material. Soluble proteins in crude extract were fractionated with ammonium sulphate and the 30-60% precipitate fraction (30-60 F) was submitted to dialysis (3,500 Da cut-offmembrane, 4°C) against the distilled water (2 h). The 30-60 F was applied (11 mg protein, hemagglutination activity) into a CM-cellulose chromatography column (5.2 cm x 1.6 cm) equilibrated with 10 mM citrate phosphate buffer pH 5.5 at flow rate of 20 mL/h. The unabsorbed proteins were eluted with the buffer solution until absorbance at 280 nm was lower than 0.05; endophytic fungal lectin was eluted with 0.5 M NaCl. Protein concentration was determined according to Lowry et al. [16] using bovine serum albumin as standard.
Lectin identification tests
Hemagglutination and hemagglutination inhibition assays: Hemagglutination tests were performed in standard microtitre platesby 2-fold serial dilution method [17]. A 50 ml aliquot of the human erythrocytes (A +ve, B +ve, AB +ve, O +ve) suspension was mixed with 50 ml of serially diluted lectin and agglutination was examined visually after incubation for 30 min at ~25°C. The unit of hemagglutinationactivity (HU) was expressed as the reciprocal of the highest dilution (titre) of the lectin that showed complete agglutination. To determine the sugar binding specificity of lectin was determined using different sugars, including D-galactose, D-mannose, D-glucose, maltose, N-acetylgalactosamine were tested for their ability to inhibit lectin induced hemagglutination. Serial two-fold dilutions of each carbohydrate were prepared in 10 mM to 100 mM range and dissolved in 0.15 M NaCl solution and mixed with equal volumes of extract containing 4 units of hemagglutination activity. Mixtures were incubated for 30 min at room temperature (26 ± 2°C), after which a suspension of humanerythrocytes (4%) was added and the whole were incubated for 1 h.The low carbohydrate concentration that produced complete inhibition of haemagglutination was determined.
Periodic Acid–Schiff staining (PAS): PAS staining was performed with slight modified method [18]. The protein separated on NATIVE-PAGE was soaked in 7.5% (v/v) aceticacid for 30 min and then with 0.2% (w/v) periodic acid for 1 h at 4°C. The periodic acid solution was removed and the Schiff reagent was added and incubated for 1 h at 4°C. Reddish-pink bands of stained glycoprotein would then be visible. The PAS reagent was removed and the gel was soaked in 7.5% acetic acid for 1 h and subsequently stored in water.
In-gel digestion of protein spots: Protein digestion was performed as described previously [19]. Spotswere excised from the stained (SDS-PAGE) gel and washed with CH3CN:H2O (1:1, v/v) containing 25 mM ammonium bicarbonate toremove the dye. The gel plug was dehydrated with 100% acetonitrile and was dried under vacuum and incubated overnight at 37°C with 20 μl of 10 μg/ml porcine trypsin in 20 mM ammonium bicarbonate. The resulting tryptic fragments were eluted by diffusion into CH3CN:H2O (1:1 v/v) and 0.5% trifluoroacetic acid. A sonic bath was used to facilitate the diffusion. The extract was vacuum dried and the pellet was dissolved in CH3CN:H2O (1:1 v/v) and 0.1% trifluoroacetic acid. The extracted tryptic peptides were used for mass spectrphotometric analysis.
In vitro antidiabetic activity of enzymes
Assay of α-amylase inhibitory activity: The effect of endophytic fungal lectin on α-amylase activity was studied using an enzyme, starch system [20]. FRB (1.5%) was mixed by stirred in 25 mL of 4% potato starch in a beaker; 100 mg of α-amylase was added to the starch solution, stirred vigorously, and incubated at 37°C for 60 min. After the incubation period, 0.1 MNaOH was added to terminate enzymeactivity. The mixture was centrifuged (3000 g; 15 min) and the glucose content in the supernatant was determined.
Assay of α-glucosidase inhibitory activity: α-glucosidase inhibitory activitywas assayed according to the method of Honda and Hara [21]. Enzyme solution (10 μL) and varying concentrations of sample emulsion (10.50 µL) were incubated together for 10 min at 37°C and the volume was made up to 210 μLwith maleate buffer, pH 6.0. The enzyme reaction was started by adding 200 µL of 2 mM p-nitrophenyl-á-d-glucopyranoside solution and further incubated at 37°C for 30 min. The reaction was terminated by treating the mixture ina boiling water bath for 5 min. After the addition of 1.0 Ml of 0.1 M disodium hydrogen phosphate solution, absorption of the liberated p-nitrophenol was read at 400 nm.
Assay of sucrase inhibitory activity: The effect of the Alternaria species lectin on sucrase activity was assayed according to the method of Honda and Hara [21]. The enzyme solution (10 μL) and varying concentrations of sample emulsion (10.50 μL) were incubated together for 10 min at 37°C and the volume was made up to 200 μL with maleate buffer (pH 6.0). The enzyme reaction was started by adding 100 μL sucrose solution (60 mM). After 30 min, the reaction was terminated by adding 200 μL of 3,5-dinitrosalicylic acid reagent and treating the mixture in a boiling water bath for 5 min. The absorbance of the solution was read at 540 nm. The percent inhibitory activities were calculated using the following formula:
% Inhibition= (AbsControl-AbsSample) x100/Abs Control
Where Abs control is the absorbance of the control reaction(containing all reagents except the test sample), and Abs sample is the absorbance of the test sample. An untreated enzyme solution was used as the control. All experiments were carried out in triplicate.
Glucose diffusion method
Amethod described by Gallagher et al.[22] was used to evaluate the effects of different solvent extracts of endophytic fungal lectin on glucose movement in vitro. This in vitro model used consisted of a dialysis tube (6 cm x 29.31 mm) (Himedia-LA393-5MT-2010) in to which 6 ml of plant extract and 2 ml of 0.15 M NaCl containing 1.65 mM D-glucose was added. The dialysis tube was sealed at each end and placed in a centrifuge tube containing 45 ml 0.15 M NaCl. The tubes were placed on an orbital shaker water bath and incubated at 37°C for 3 h. The movement of glucose into the external solution was provided. Concentrations of glucose within the dialysis tube were measured and control tests were conducted in the absence of plant extracts. Glucose concentrations were analyzed by enzymatic method using glucose oxidase kit. All tests were carried out in triplicate and the results were presented as means ± SD.
In vivo experiments
Selection of animal species: Healthy young male albino wistar rats of 8-10 week old, weighing between 150 g to 200 g were selected for in vivo antidiabetic studies. Rats were collected from the animal house of Sree Siddaganga College of Pharmacy, Tumkur, Karnataka, India and research was carried out in Phamacology Department of Sree Siddaganga College of Pharmacy. Rats were housed in animal room at 25 ± 2ºC temperatures and maintained with free access to standard food and pure water ad libitum. The animal room was regulated by a 12 h light and 12 h dark schedule.
Toxicity studies (LD50): Based upon the results of in vitro antidiabetic activity, the endophytic fungal Alternaria species lectin was selected for animal studies. Suspensions of dried, concentrated column fractions were prepared by dissolving in 0.9% v/v cold normal saline solution for treatment. Toxicity studies were carried out endophytic fungal Alternaria species lectinin accordance with the modified method Lorke [23]. Maximum dose, up to 1200 mg/kg body weight was treated through oral route of administration. The animals were grouped into 4 groups involving 5 animals in each group. Group-1: lectin- 400 mg/kg body weight, Group-2: lectin fraction- 800 mg/kg body weight, and Group-3: lectin- 1200 mg/kg body weight. The rats were observed for clinical signs and symptoms of toxicity like behavioural changes and mortality within 24 h. All the procedures were performed in accordance with the Institutional Animal Ethics Committee (IAEC). Lethal dose - 50 (LD50) was then calculated as the square root of the product of the lowest lethal dose and high non-lethal dose.
Experimental induction of diabetes
The animals were fasted for 16 - 18 h with free access to water prior to the administration of Alloxan. Alloxan monohydrate is cyclic urea analogue having unique properties of producing chronic experimental diabetes by a specific cytotoxic action on β-cells of the islets of Langerhans by a single diabetogenic dose. Diabetes was induced in nearly 70 rats by intraperitoneal (i.p.) injection of Alloxan monohydrate at a dose of 120 mg/kg body weight, dissolved in 0.9% v/v cold normal saline solution [24]. The rats were then kept for the next 24 h on 5% glucose solution bottles in their cages to prevent hypoglycemia [25]. After an observational period of about 72 h, rats with fasting plasma glucose levels above 300 mg/dl were considered diabetic.
Experimental protocol: After an observational period of 72 h, rats with fasting plasma glucose levels above 300 mg/dl were considered as diabetic and were assigned into five groups of ten rats in each group.
Animal fasted overnight were randomly divided into 5 groups:
|
Group 1:
|
NC:
|
Normal control, treated with only normal saline (2.0 ml) orally.
|
|
Group 2:
|
DC:
|
Diabetic control
|
|
Group 3:
|
STD:
|
Diabetic rats treated with reference drug Glibenclamide, at a dose of 0.5 mg/kg body weight.
|
|
Group 4:
|
EAsL:
|
Diabetic rats treated with lectin isolated from Alternaria species, at a dose of 400 mg/kg body weight orally.
|
The fasting blood glucose levels were estimated by glucose oxidase-peroxidase reactive strips (Dextrostix, Bayer Diagnostics) with Accu-chek glucometer. Blood samples were collected by cutting the tip of the tail at an interval of 1, 7, and 14 days. The blood results were reported as mg/dl. Blood glucose levels were expressed in mg/dl as mean ± SEM. On day 14, rat’s body weight, serum cholesterol and serum triglycerides were analysed.During the experiment all the rats had free access to standard rat chow and water at all times. Body weight and glycaemic change were calculated according to the formula;
|
Change in body weight (bw) (%) =
|
(body weight on 14th day – Initial body weight) x 100
|
|
Initial body weight
|
|
% Glycaemic change =
|
(Glucose concentration 14th day – Fasting blood glucose on 1st day) x 100
|
|
Fasting blood glucose on 1st day
|
The data were statistically analyzed using ANOVA with multiple comparisons versus control group. Values of p<0.05 or less were taken as significant.
Lipid profile
At the end of 14th day of experiment period, blood was collected in Eppendorf tubes through retro-orbital plexus. Plasma was separated and serum was taken by centrifugation at 4ºC using REMI-24 model centrifuge. Lipid profile viz., serum cholesterol and serum triglycerides were measured on automated analyser Olympus AU 400.
Histopathology
It is been important to study the determination of affects of endophyticlectin on pancreatic cytoarchitecture. At the end of 14th day, all the animals were sacrificed by lethal chloroform vapor anaesthesia and pancreas was excised and rinsed in ice cold normal saline. A portion of the tissue was fixed in 10% formalin, cut into 5 μm thick sections, and stained using hematoxylin-eosin stain and histopathological observations were made.
Effect of endophyticlectin on biochemical data
Normal control, diabetic induced control, lectin treated and standard drug treated rats blood samples were centrifged at 2,500 g for 15 min at 4°C (REMI, C 24 Cooling Centrifuge, India). Sera were obtained and the levels of the urea, creatinine, aspertate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by enzymatic colorimetric methods in a chemistry autoanalyzer [12].
Molecular characterization of antidiabeticlectin by MALDI-TOF-MS
The dried extract was dissolved in 10 μl of 0.1% trifluoroacetic acid (TFA), purified by Zip Tip C18 tips prior to MS analysis. The elutant was mixed with 10 mg ml-1 of 2, 5- dihydroxycinnamic acid (DHB) in 50% acetonitrile and 0.1% TFA for spotting onto sample plate anddried for MALDI-TOF-MS analysis. MALDI-TOF-MS analysis wasperformed with the 4700 proteomics analyzer (Applied Biosystems, Framingham, MA, USA) equipped with an ND:YAG laser (355 nm) having a 3–7 ns pulse and a 200 Hz firing rate. MALDI-TOF-MS analyses were obtained in the reflectron positive ion mode. The accelerated voltage of the ion source for MS and MS/MS analyses was set at 20 and 8 kV, respectively peaks were detected using the internal algorithm of the 4000 series software with the signal-to-noise ratio parameter set at 30 for MS, analysis was done at IISc, Bangalore, India [26].
Five different endophytic fungal species were identified from three (stem, leaves and fruit) parts of Viscum album [26] from our earlier report. The Alternaria species was present in all the three parts used. For the present study, the leaf endophytic fungi Alternaria species was selected for present study and it was found to confirmed to contain heamagglutinate protein in agglutinating method, at 25 mM and confirms the lectin is a D-glucose and D-galactosamine specific. We have selected D-galactosaminelectin and in molecular weight determination showed a single band of 64 kDa [26]. The lectin was stained purplish pink with Schiff’s reagent in NATIVE-PAGE in PAS and it confirms as glycoprotein lectin.
The lectin had shown significant inhibition effects on glucose movement into external solution across the dialysis membrane. The lectin showed 41.37 ± 0.59 of glucose diffusion in dialysis membrane and increment movement was 12.54 ± 0.87% (Table 1).
Table1. Effect of lectin extract on glucose diffusion from dialysis tube after 3h
Extracts |
Glucose diffusion to out of dialysis membrane |
Increase of movement (%) |
Lectin |
41.37 ± 0.59 |
12.54 ± 0.87 |
*Repeated the experiment thrice, data represents mean ± SD of three replicates
*p<0.01 (plese show significant data for lectin effect)
The α-amylase inhibitory activity of lectin was studied using α-amylase star remodel system and inhibitory activity was 85.26 ± 1.25. The lectin significantly inhibited the α-glucosidase and it was 93.41 ± 1.27. The significant enhancement in inhibitory activity of α-glucosidase by lectin. More sucrase inhibition was noticed in lectin (81.61 ± 1.05%) (Figure 1).

Figure 1. Effect of lectinon α-amylase, α-glucosidase and sucrase activity
Toxicity studies (LD50)
The fungal endophyte, Alternaria species lectin was used for toxicity studies in rats through oral route administration. No toxicity was observed with fungal lectin in rats upto 1200 mg/kg body weight. We have also studied clinical signs and symptoms of toxicity like behavioural changes include locomotary activity and sensitivity touch and mortality within 24 h and also recorded after 48 h of administration. The endophytic fungal lectin did not cause deaths of any abnormal behavior changes in rats and 100% survival rates had seen. The endophytic lectin was considered as non-toxic in test concentrations.
Effect on body weight
There is a slight increase in body weight in normal control and it may be due to normal growth. The diabetic control rats had reduced the body weight of 5.28%. The diabetic induced rats treated with standard drug Glibenelamide increased in the body weight by 9.01%. The lectin treated rats increased the body weight by 8.50% significantly (Table 2).
Table 2. Effect of lectinextract on average body weight of diabetic induced rats
Group |
Dose (mg/kg bw) |
Average body weight (gm) |
Day 1 |
Day 7 |
Day 14 |
Change in bw (%) |
|
Group 1: |
Normal saline |
192.34 ± 3.21 |
203.54 ± 3.05 |
209.53 ± 5.31 |
8.20 |
|
Group 2: |
120 mg/kg body weight |
198.14 ± 4.52 |
181.56 ± 2.82 |
175.81 ± 4.49 |
-11.26 |
|
Group 3: |
0.5 mg/kg body weight |
203.03 ± 3.44 |
210.23 ± 3.61 |
221.33 ± 5.02 |
9.01 |
|
Group 4: |
40 mg/kg body weight |
193.05 ± 3.09 |
199.59 ± 4.14 |
209.47 ± 4.69 |
8.50 |
|
*Rrepeated the experiment thrice, data represents mean ± SD of three replicates
Note: Group 1: Normal control, Group 2: Diabetic control, Group 3: Diabetic rats treated with standard Glibenclamide and Group 4: Diabetic rats treated with Lectin extract
*p<0.01 (plese show significant data for lectin effect)
Hypoglycaemic effect of endophytic fungal lectin
The Alternaria species fungal lectin had significant hypoglycaemic effects. The blood glucose level was decreased and observed by 60.76% on 14th day at 400 mg/kg concentration. The blood glucose level reduction was found to be more efficient in lectin treated when compared to standard drug Glibenclamide which shown blood glucose level by 64.04%. The blood glucose on the final day of treatment were 101.33 ± 4.46 mg/dl (normal control), 386.44 ± 10.48 mg/dl (diabetic control), 115.34 ± 5.45 mg/dl (diabetic rats were treated with standard drug Glibenclamide) and 129.62 ± 9.10 mg/dl (lectin treated rats) (Table 3).
Table 3. Effect of lectinextracton blood glucose level of diabetic induced rats
Group |
Dose (mg/kg bw) |
Blood glucose concentration (mg/dl) |
Day 1 |
Day 7 |
Day 14 |
Glycaemic change (%) |
Group 1: |
Normal saline |
98.40 ± 5.01 |
96.14 ± 4.50 |
101.33 ± 4.46 |
2.97 |
Group 2: |
120 mg/kg bw |
323.03 ± 09.10 |
352.14 ± 11.50 |
386.44 ± 10.48 |
19.62 |
Group 3: |
0.5 mg/kg bw |
320.80 ± 10.45 |
209.46 ± 09.52 |
115.34 ± 05.45 |
-64.04 |
Group 4: |
400 mg/kg bw |
3302021 Copyright OAT. All rights reserv
| 215.46 ± 8.41 |
129.62 ± 09.10 |
-60.76 |
*Repeated the each experiment thrice, data represents mean ± SD of three replicates
Note: Group 1: Normal control, Group 2: Diabetic control, Group 3: Diabetic rats treated with standard Glibenclamide and Group 4: Diabetic rats treated with Lectin extract
*p<0.01 (plese show significant data for lectin effect)
Effect of endophyticlectin on biochemical data
Normal control, diabetic (induced) control, lectin treated and standard drug treated rat blood samples were centrifuged at 2,500 g for 15 min at 4°C (REMI, C 24, Cooling centrifuge, India). Sera were obtained and the levels of the urea, creatinine, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by enzymatic colorimetric methods in a chemistry autoanalyzer (Rocha et al., 2013).
After treatment of 14 days, the rats were subjected to identify serum cholesterol and serum triglycerides and noticed diferent levels of each. The lectin treated rats showed serum cholesterol (mg/kg bw) and serum triglycerides by 90.04 ± 0.92 and 98.51 ± 2.02 respectively. The diabetic induced had shown serum cholesterol and serum triglycerides by 153.26 ± 1.45 and 176.64 ± 2.62 respectively. The lectin treated diabetic rats showed reduced levels of serum cholesterol (103.54 ± 2.13) and serum triglycerides (124.68 ± 2.49) whereas standard glibeneclamide showed 98.44 ± 1.28 and 110.44 ± 2.02 of serum cholesterol and serum triglycerides (Figure 2).
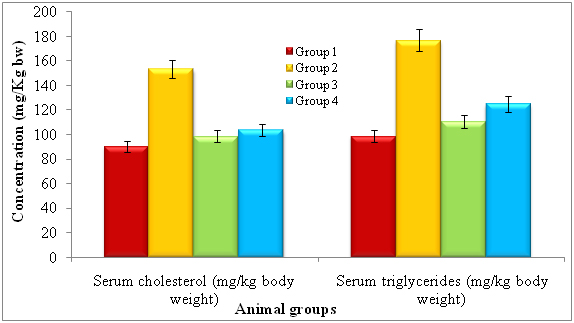
*Repeated the each experiment thrice
Note: Group 1: Normal control, Group 2: Diabetic control, Group 3: Diabetic rats treated with standard Glibenclamide and Group 4: Diabetic rats treated with lectin extract.
*p<0.01 (please show significant data for lectin effect)
Figure 2. Effect of lectinextract on serum lipid profilein diabetic induced rats at the end of 14th day
Histopathology
The results of lectin treated rats exhibited well differentiated by cytoarchitecture. In normal control, there was normal distribution of cells of islets, acini, blood capillaries, pancreatic ducts were observed. The normal control also showed acinar cells, islets of Langerhans and blood vessels were good in number, texture and were well organized and connected with connective tissues. Along with RBCs, the 2-3 types of distinguishable islet cells were noticed. No inflammatory pancreatic and blood vessels were seen. Noticed abnormalities were observed in diabetic induced rats and marked inflammatory of blood vessels and ruptured blood capillaries were observed. Pancreatic lobes, acini, islets of Langerhans, exocrine glands, connective tissues and capillaries were depleted and number, cells of islets and pancreatic ducts were decreased. Deformed islets were observed due to diabetes effect and they were formed as amyloid and shrinkage. In endocrine and exocrine, the red blood cells were interdispersed (Figure 3A-3B).
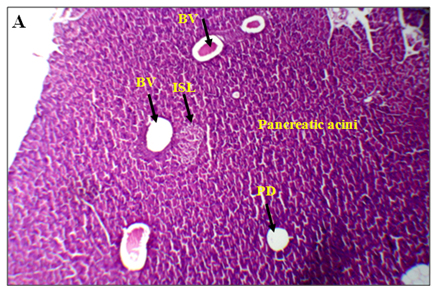
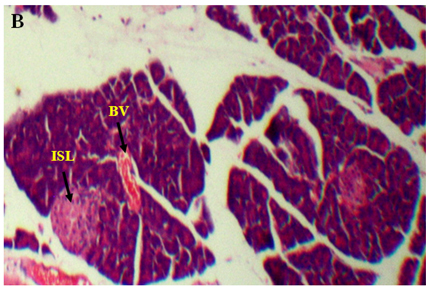
Figure 3. An overview of histopathology of rat pancrease, A) Diabetic control with inflammatory blood vessels and islet cell damage B) Lectin treated rat pancrease cells (BV-Blood vessel, PD-Pancreatic duct, ISL-Islet of Langerhans)
The standard drug, glibenclamide treated diabetes rats had shown regeneration of pancreatic ducts, acinar and connective cells. The interlobular septa, walls of blood vessels, and pancreatic ducts were normal as in normal control. Even distribution of islets and cells of Langerhans, cells of acinar and pancreatic ducts had seen and they were normal (Figure 4A-4B). The endophytic fungi, Alternaria species lectin treated diabetes induced rats exhibited significant in preventing damage of pancreatic cells by oxidation stress. The well organized in accordance with their number, texture and dispersion of pancreatic connective tissue and cells, acini, blood vessels and capillaries, pancreatic ducts and their walls, islets capsule had noticed in lectin treated rats. Distinguishable individual of cells of islets and noticed the presence of 2-3 types of islet cells with RBC cells. The architecture of pancreatic histology in lectin treated diabetic induced rats is normal and was similar to normal control (Figure 5A-5F).
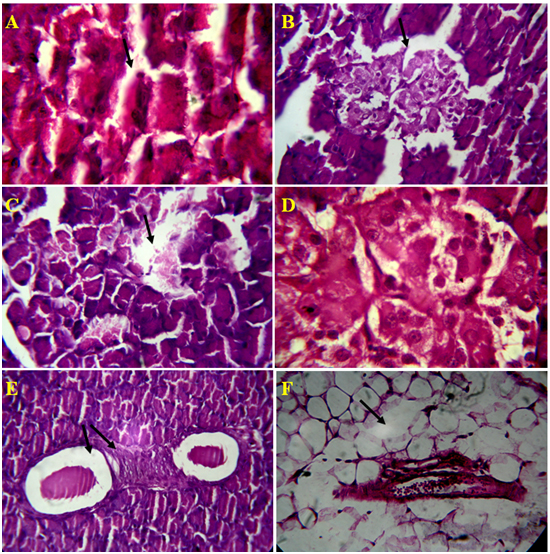
Figure 4. Microscopic views of Alloxan induced rat pancreatic tissue, A) Damage ofAcini cell lining, B) Rupture of islet cells and it`s wall, C) Depletion Islet of Langerhansand exocrine cells, D) Rupture of capillaries inIslet of Langerhans, E) Inflammatory blood vessels, F) Depletion of connective tissue around pancreatic duct and blood vessel in 4 µm section
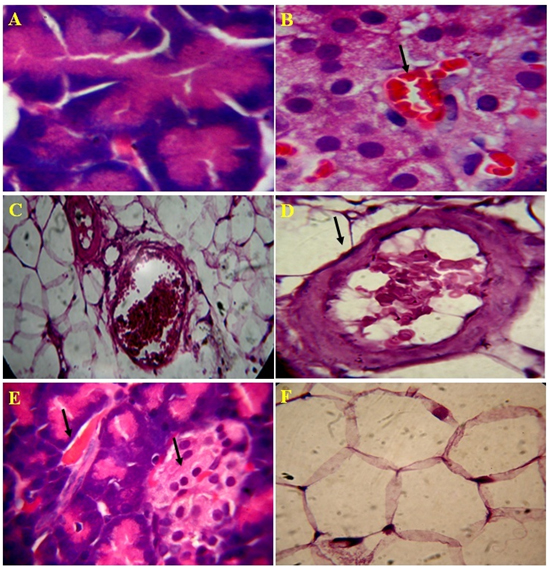
Figure 5. Microscopic views of the effect of Lectin extract on rat pancreatic tissue, A) Normal acinar cells, B) Recovery of islet cells and blood cells, C) improved connective tissue around pancreatic duct and blood vessel, D) Recovered blood vessel and proper connective tissue E) Improvised blood vesel and islets of Langerhans and F) Normal flow of connective tissue in 4 µm section
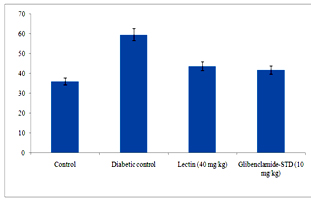

Figure 6. Serum urea (A) and creatinine (B) levels in diabetic rats after treatment of 14 days with endophytic fungal lectin
*p<0.01 (please show significant data for lectin effect)
Figure 6 clearly depicts that different levels of urea and creatinine known as kidney function markers were significantly increased in sera of alloxan induced diabetic rats. The levels of urea and creatinine significantly decreased after 14 days of treatment. The lectin (40 mg/kg) treated rats had reduced the serum level of urea and creatinine by 15.9% and 0.08% respectively.
The serum AST and ALT were significantly reduced in lectin treated alloxan induced diabetic rats after treatment with 40 mg/kg. The AST and ALT reduced in lectin treated rats by 26% and 5% respectively (Figure 7 and 8).
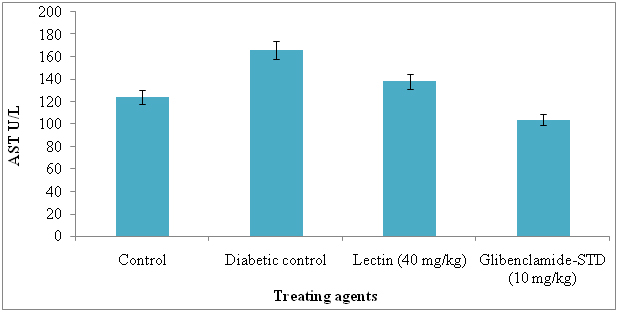
Figure 7. Serum aspertate aminotransferase levels in diabetic rats after treatment of 14 days with endophytic fungal lectin
*p<0.01 (please show significant data for lectin effect)
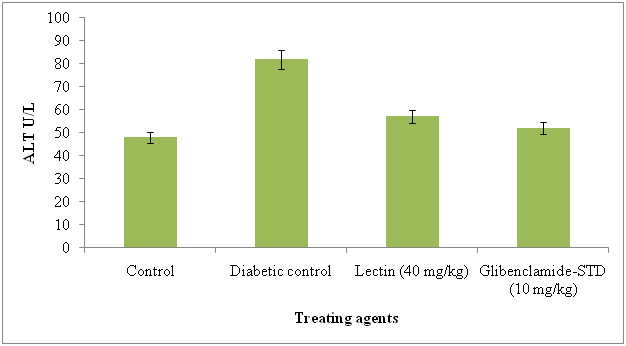
Figure 8. Serum alanine aminotransferase levels in diabetic rats after treatment of 14 days with endophytic fungal lectin
*p<0.01 (please show significant data for lectin effect)
MALDI-TOF-MS analysis of Alternaria species lectin showed the presence of 8 different peptides,of which, 7 peptides had maximum intensities. The peptide fingerprinting of lectin from Alternaria species was identified as a ribosome inactivating protein comparable to Phytolacca americana. The seven peptides were 2100.09, 1716.86, 1365.72, 1838.95, 893.51, 2329.15, 1179.65, 1765.81 and matched peptides. The protein identified was similar to Phytolacca americana reported to have antiviral activity, they belong to ribosome inactivating protein family.
The lectin isolated from endophytic fungi, Alternaria species of Viscum album and characterized by hemagglutination and it was a 64 kDa from our earlier lectin had shown very potent inhibitor of important diabetic enzymes tested viz., - amylasereports are confirmatory findings of mentioned references. The glucose diffusion results were confirmation with the findings of tried with other different extracts which were potent inhibitors of glucose movement in the same model system and the ability to inhibit glucose diffusion using same in vitro method with different plant extracts.
Some antidiabetic plants may exert their action by stimulating the function or number of β-cells and increase the insulin release. In some other plants, the effect is due to decreased blood glucose synthesis due to the decrease in the activity of enzymes. In other plants, the activity is due to slow absorption of carbohydrates and inhibition of glucose transport [31].
The enzyme, α-amylaseis responsible in hydrolyzing dietary starch into maltose which then breaks down to glucose prior to absorption. Sinceá-amylases play an important role in starch assimilation in human beings and animals, the presence of such inhibitors in foodstuffs or plant extracts may be responsible for impaired starch digestion [32]. α-amylase inhibitors may be of value as novel therapeutic agents [33].
The α-glucosidase are crucial in many biological processes includes break down of edible carbohydrates and are also involved in a variety of metabolic disorders such as diabetes [34]. Thus potent and selective glucosidase inhibitors have many interesting potential applications, especially to manage in diabetes [34]. α-glucosidase is one among a number of glucosidases located in the brush-border surface membrane of intestinal cells and is a key enzyme of carbohydrate digestion. α-glucosidase inhibitors block the action of enzyme in the small intestine,which is rate limiting in the conversion of oligosaccharides to monosaccharides necessary for gastrointestinal absorption. Postprandial glucose peaks may be attenuated by delayed the glucose absorption. The main benefits attributed to α-glucosidase inhibitors are, reduction in both postprandial glycemic levels and the total range of postprandial glucose levels [35].
After treatment with lectin to Alloxan induced rats noticed that had normal body weight, blood glucose level, serum lipid profile and normal pancreatic histological characters after 14 days also. The rats body weight were decreased as diabetes progresses [36]. The lectin treated rats body weight were significantly increased, the results were correlated with the findings of Ahmed et al. [37] and Channabasava et al. [38] (unpublished data).
Saravanan et al. [39] and Quiong et al. [40] have reported that Alloxan induced diabetes mellitus rats had shown hyperglycemia and hyperlipidemia. Marles and Fornsworth [41] and Grower et al. [42] had used plant extracts were responsible for reducing blood glucose level. The endophyticlectin had significant hyperglycemia and antihyperglycemic activity after 14 days of treatment. The similar results have been reported by Harikiran et al. [43] with Bombax melabaricum, Tyagi et al. [44] with Anacyclus pyrethrum, Channabasava et al. [38] with Loranthus micranthus extract (unpublished data). The lectin isolated from Urtica pilulifera seeds had shown similar activity [45]. In hyperlipidemia, serum triglycerides play an important role of development and progression of renal diseases in type I diabetes mellitus [46]. Inactivation and derangements of lipid metabolism are vital determinants and status of the diabetes [47].
In lectin treated diabetic rats had shown significantly decrease in hyperlipidemia parameters by observing elevated serum cholesterol and serum triglycerides. Lectin have shown similar activity was noticed by Liang et al. [48] with Hericium erinaceus.
Alloxan destroys β-cells of islets of pancreas, it elevatesthe blood glucose level, decrease the protein content, increases the level of cholesterol and triglycerides. In almost all antidiabetic activity, the Alloxan is commonly used and it is similar to diabetes patient [49]. Alloxan producing redox potential and forms superoxide radicals and these increases concentrations of cytosolic calcium destruct the β-cells rapidly [50]. In diabetic induced rats are usually shown decrease in cell numbers in islets, cell damage and cell death [38,51]. The blood vessels were changed into thickened and hyalinized and it causes hypoxia and results into changes in degeneration and necrosis [52] and disorganization of pancreatic architecture and insulin producing cells were depletion leads to structural and functional alterations in Alloxan induced rats [38,53].
The endophytic fungal lectin induced the regeneration of connective tissues, ducts and walls of pancreatic, acini, blood vessels, blood capillaries, islet capsules and cells and organization, number, texture and dispersion were changed in Alloxan induced rats [38,54]. The lectin was also showed β-cells regeneration and blood glucose decrease [38]. The reduction of urea and creatinine was observed in lectin treated diabetic rats. Our results are in agreement with recent reports of Rocha et al. [12] and Omara et al. [55] but they used different plant secondary metabilites.
Significant reduction in AST and ALT in lectin treated rats has an evidence of good health. Our results are in agreement with those of Mansour et al. [56] and Rocha et al. [12] and they reduced these by using different plant metabolites. The levels of serum AST and ALT reduction in diabetic induced rats was due to effect of lectin. The liver problems or diseases are a high problem of health world wide and release of intracellular localized marker enzymes such as AST and ALT into the blood, when cell and mitochondria suggested to injury indicates hepatocytes damage [57]. In MALDI-TOF-MS, the N-acetyl-galactosaminelectin bearing 64 kDa molecular weight lectin was identified and it was similar to ribosomal inactivate protein of Phytolacca americana. The seven peptides were identified in lectin and they were, 2100.09, 1716.86, 1365.72, 1838.95, 893.51, 2329.15, 1179.65, 1765.81 and matched peptides and it was similar to lectin II group and they present in P. Americana and possessing ribosomal inactivation and antiviral activity. Now we report here that same lectin is exhibiting strong antidiabetic activity in alloxan induced diabetes rats.
All the three diabetic enzymes were inhibited by lectin in in vitro and regeneration of histopathological structures in Alloxan induced rats in in vivo had showed protective in effect by altering in structural and functional changes in ducts of pancreatic, β-cells, islet, acini. Here, we report first in nationally and internationally that, lectin isolated from endophytic fungi Aspergillus species was shown strong antidiabetic activity in both in vitro and in vivo. The lectin reduced the levels of urea, creatinine, serum cholesterol, triglycerides, aspertate aminotransferase and alanine aminotransferase in diabetes induced rats. In MALDI-TOF-MS, the N-galactosaminelectin bearing 64 kDa molecular weight glycoprotein was identified and it was similar to ribosome inactivate protein of Phytolacca americana and which shown strong antidiabetic activity. Further, this endophytic fungal lectin can be used for diabetes treatment.
We thank Dr. MR Hulinaykar, Managing Trustee, Sri Shridevi Charitable Trust (R.), Dr. S. M. Shashidhara, Principal and SreeSiddaganga College of Pharmacy, Tumakuru, Karnataka, India and Molecular biophysical unit, IISc, Bangalore, Karnataka India, for encouragement and suggestions during the research. We also thank, Dr Prasad S Koka is a Ramalingaswami Fellow of the Department of Biotechnology, Government of India, New Delhi.
- Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67: 257-268. [Crossref]
- Gunatilaka AAL (2006) Natural products from plant associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69: 505-526. [Crossref]
- Verma VC, Kharwar RN, Strobel GA (2009) Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat Prod Commun 4: 1511-1532. [Crossref]
- Rodriguez RJ1, White JF Jr, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182: 314-330. [Crossref]
- Xu L, Zhou L, Zhao J, Jiang W (2008) Recent studies on the antimicrobial compounds produced by plant endophytic fungi. Natural Product Research and Development 20: 731-740.
- Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, et al. (1999) Discovery of small molecule insulin mimetic with antidiabetic activity in mice. Science 284: 974-981. [Crossref]
- Prabhavathy D, Nachiyar V (2013) Antimicrobial and antidiabetic activity of an endophytic fungi isolated from Adathodabeddomei. Int J Pharm Pharm Sci 5(3): 780-783.
- Edward DJ, Srikandace Y, Suharso WP, Cahyana H, Simanjuntak P (2011) Potential endophytic microbes selection for antidiabetic bioactive compounds production. Asian Journal of Biochemistry 6: 465-471. [Crossref]
- Nowell PC (1960) Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res 20: 462-466. [Crossref]
- Li Q, Ye XL, Zeng H, Chen X, Li XG (2012) Study on the extraction technology and hypoglycemic activity of lectin from Trichosanthes kirilowi. Zhong Yao Cai 35: 475-479. [Crossref]
- Hemalatha C, Dhamotharan R, Murugesan S (2011) Effect of Soya bean lectin on streptozotocin induced diabetic rats. Asian J Exp Biol Sci 2(2): 231-236. [Crossref]
- Rocha AMD, Araújo TFDS, Fonseca CSMD, Mota DLD, Medeiros PLD, et al. (2013) Lectin from Crataeva tapia bark improves tissue damages and plasma hyperglycemia in Alloxan-induced diabetic mice. Evidence-Based Complementary and Alternative Medicine ID 869305.
- Vessby B (2000) Dietary fat and insulin action in humans. Br J Nutr 83 Suppl 1: S91-96. [Crossref]
- Seidell JC (2000) Obesity, insulin resistance and diabetes--a worldwide epidemic. Br J Nutr 83 Suppl 1: S5-8. [Crossref]
- Araújo WL, Marcon J, Maccheroni W Jr, Van Elsas JD, Van Vuurde JW, et al. (2002) Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68: 4906-4914. [Crossref]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Moreira RA, Oliveira JTA (1983) Lectins from the genus Artocarpus. Biologia Plantarum 25: 343-348.
- Dubray G, Bezard G (1982) A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem 119: 325-329. [Crossref]
- Natarajan SS, Xu C, Bae H, Caperna TJ, Garrett WM (2006) Characterization of storage proteins in wild (Glycine soja) and cultivated (Glycine max) soybean seeds using proteomic analysis. J Agric Food Chem 54: 3114-3120. [Crossref]
- Ou S, Kwok K, Li Y, Fu L (2001) In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem 49: 1026-1029. [Crossref]
- Honda M, Hara Y (1993) Inhibition of rat small intestinal sucrase and á-glucosidase activities by tea polyphenols. Bioscience Biotechnology Biochemistry 57: 123-124.
- Gallagher AM, Flatt PR, Duffy D, Adbel-WahadYHA (2003) The effects of traditional anti-diabetic plants on in vitro glucose diffusion. Nutraceutical Research 23: 413-424.
- Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54: 275-287. [Crossref]
- Katsumata K, Katsumata Y, Ozawa T, Katsumata K Jr (1993) Potentiating effects of combined usage of three sulfonylurea drugs on the occurrence of alloxan diabetes in rats. Horm Metab Res 25: 125-126. [Crossref]
- Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N (2002) Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res 46: 251-255. [Crossref]
- Sadananda TS, Govindappa M, Vinay DG, Bhawna B, Prahlad Baishya, et al. (2014) Isolation and characterization of antiviral and ribosome inactivating protein from the endophytic fungi Alternaria sp from Viscum album using MADLI-TOF-MS and their antibacterial activity. Drug Invention Today 6: 105-112.
- Virounbounyapat P, Karnchanatat A, Sangvanich P (2012) An alpha-glucosidase inhibitory activity of thermostable lectin protein from Archidendron jiringa Nielsen seeds. Afr J Biotechnol 11: 10026-10040.
- Moreno J, Altabella T, Chrispeels MJ (1990) Characterization of alpha-Amylase-Inhibitor, a Lectin-like Protein in the Seeds of Phaseolus vulgaris. Plant Physiol 92: 703-709. [Crossref]
- Upadhyay SK, Singh PK (2012) Receptors of garlic (Allium sativum) lectins and their role in insecticidal action. Protein J 31: 439-446. [Crossref]
- Büyükbalci A1, El SN (2008) Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods Hum Nutr 63: 27-33. [Crossref]
- Chhetri R, Basnet D, Chiu PF, Kalikotay S, Chhetri G, et al. (2005) Current status of ethnomedicinal plants in the Darjeeling, Himalaya. Current Science 89: 268-268.
- Reddy NVLS, Anarthe SJ, Raghavendra NM (2010) In vitro antioxidant and antidiabetic activity of Asystasia gangetica (Chinese Violet) Linn. (Acanthaceae). International Journal of Research in Pharmaceutical and Biomedical Sciences 1: 72-75.
- Puls W, Keup U (1973) Influence of an -amylase inhibitor (BAY d 7791) on blood glucose, serum insulin and NEFA in starch loading tests in rats, dogs and man. Diabetologia 9: 97-101. [Crossref]
- Robinson KM, Begovic ME, Rhinehart BL, Heineke EW, Ducep JB, et al. (1991) New potent alpha-glucohydrolase inhibitor MDL 73945 with long duration of action in rats. Diabetes 40: 825-830. [Crossref]
- Truscheit E, Frommer W, Junge B, Muller L, Schmidt DD, et al. (1981) Chemistry and biochemistry of microbial a-glucosidase inhibitors. Chem Int Ed Engl 20: 744-761.
- Kamanna VS, Roh DD, Kirschenbaum MA (1998) Hyperlipidemia and kidney disease: concepts derived from histopathology and cell biology of the glomerulus. Histol Histopathol 13: 169-179. [Crossref]
- Ahmed MF1, Kazim SM, Ghori SS, Mehjabeen SS, Ahmed SR, et al. (2010) Antidiabetic Activity of Vinca rosea Extracts in Alloxan-Induced Diabetic Rats. Int J Endocrinol 2010: 841090. [Crossref]
- Channabasava, Govindappa M, Chandrappa CP, Umashankar T, Sadananda TS (2015) GC-MS study of two column fractions from methanol extracts of Loranthus micranthus and their in vivo antidiabetic activity on alloxan induced diabetic rats. [Unpublished]
- Saravanan G, Pari L (2003) Effect of Cogent db, a herbal drug, on serum and tissue lipid metabolism in experimental hyperglycaemic rats. Diabetes Obes Metab 5: 156-162. [Crossref]
- Luo Q, Cai Y, Yan J, Sun M, Corke H (2004) Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci 76: 137-149. [Crossref]
- Marles RJ, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2: 137-189. [Crossref]
- Grover JK, Yadav S, Vats V (2002) Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol 81: 81-100. [Crossref]
- Harikiran L, Swapna CH, Someshwar K, Rama G, Krishna K, et al. (2011) Antihyperglycemic effects of Bombax malabaricum extracts in Alloxan induced diabetic rats. Der Pharmacia Sinica 2: 74-80.
- Tyagi S, Mohd. Mansoori H, Singh NK, Shivhare MK, Bhardwaj P, et al. (2011) Anti-diabetic effect of Anacyclus pyrethrum DC in Alloxan induced diabetic rats. European Journal of Biological Sciences 3: 117-120.
- Kavalali G, Tuncel H, Göksel S, Hatemi HH (2003) Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J Ethnopharmacol 84: 241-245. [Crossref]
- Hadjadj S, Duly-Bouhanick B, Bekherraz A, BrIdoux F, Gallois Y, et al. (2004) Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab 30: 43-51. [Crossref]
- Bach JF (1997) Autoimmunity and type I diabetes. Trends Endocrinol Metab 8: 71-74. [Crossref]
- Liang B, Guo Z, Xie F, Zhao A (2013) Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement Altern Med 13: 253. [Crossref]
- Dhanabal SP, Raja MK, Ramanathan M, Suresh B (2007) Hypoglycemic activity of Nymphaea stellata leaves ethanolic extract in alloxan induced diabetic rats. Fitoterapia 78: 288-291. [Crossref]
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50: 537-546. [Crossref]
- Dahech I, Belghith KS, Hamden K, Feki A, Belghith H, et al. (2011) Oral administration of levan polysaccharide reduces the alloxan-induced oxidative stress in rats. Int J Biol Macromol 49: 942-947. [Crossref]
- Pushparaj P, Tan CH, Tan BK (2000) Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol 72: 69-76. [Crossref]
- Hashemi M, Dostar Y, Rohani SR, Saraji AR, Bayat M (2009) Influence of aloxane on the apoptosis of pancreas ß-cells of rat. World J Med Sci 4: 70–73.
- Stanley Mainzen Prince P, Kamalakkannan N (2006) Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J Biochem Mol Toxicol 20: 96-102. [Crossref]
- Omara EA, Nada SA, Farrag AR, Sharaf WM, El-Toumy SA (2012) Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine 19: 1059-1067. [Crossref]
- Mansour HA, Newairy AS, Yousef MI, Sheweita SA (2002) Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 170: 221-228. [Crossref]
- Kumar Rajagopal S, Manickam P, Periyasamy V, Namasivayam N (2003) Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem 14: 452-458. [Crossref]










