Abstract
The development of cutaneous mammary vascular lesions following irradiation as breast cancer therapy has been well-documented. Accurately distinguishing between atypical vascular lesions (AVL) and angiosarcoma (AS) is of great clinical consequence, as their treatment and prognosis differ greatly. Exact classification based on histology alone on a small biopsy sample has the potential to be quite challenging, as areas of AS may be histopathologically indistinguishable from AVL. Studies have confirmed MYC amplification in secondary AS, and found lack of amplification in radiation-induced AVL. Furthermore, anti-MYC IHC has been shown to be a sensitive marker in AS, and could serve as a useful diagnostic tool for pathologists. This review article describes and compares the clinical and histologic features of AVL and radiation-associated AS, with special emphasis on advances made in the understanding of the genetic basis of these lesions as well as the utility of ancillary testing to aid in this challenging differential diagnosis.
Key words
secondary angiosarcoma, atypical vascular lesion, MYC amplification, radiation-induced angiosarcoma
Introduction
The development of cutaneous mammary vascular lesions following irradiation as breast cancer therapy has been well-documented. Accurately distinguishing between atypical vascular lesions (AVL) and angiosarcoma (AS) is of great clinical consequence, as their treatment and prognosis differ greatly. Exact classification based on histology alone on a small biopsy sample has the potential to be quite challenging, as areas of AS may be histopathologically indistinguishable from AVL. This was best illustrated in a study by Mattoch et al. [1] in which approximately 50% of cases diagnosed as AVL were reclassified as AS upon examination of an excisional specimen. As treatment paradigms have continued to shift from radical mastectomy with lymph node dissection to breast-conserving surgery with adjuvant radiation therapy, dermatopathologists are likely to encounter biopsies of vascular lesions occurring in this setting with increasing frequency.
This article reviews and compares the clinical and histologic features of AVL and radiation-associated AS, with special emphasis on advances made in the understanding of the genetic basis of these lesions as well as the utility of ancillary testing to aid in this challenging differential diagnosis.
Angiosarcoma
Secondary angiosarcoma is a potentially aggressive malignancy that is most commonly seen in patients treated for breast carcinoma. Development of AS subsequent to radical mastectomy and axillary lymph node dissection was first described in 1949 by Stewart and Treves [2], and is generally accepted to be due to surgically-induced chronic lymphedema. Breast-conserving surgery with adjuvant radiation has since been successful in regards to quality of life, cosmetic outcome, and long-term survival [3-5]. As a result, lymphedema-associated AS is now rare in breast cancer patients, and there appears to be an increasing incidence in post-radiation AS in this patient population [6-9]. Primary angiosarcoma of the breast is rare and arises without a recognized association factor, usually within the breast parenchyma, and affects a younger population of women in their 20s – 40s [10-12].
Though rare, with a cumulative incidence of 0.9 per 1000 cases during 15 years, women treated with radiation therapy have a more than 1000-fold increased relative risk of developing cutaneous AS compared with the general population. [7,13]. Billings et al. [6] found a median interval to diagnosis of 59 months, roughly half the time interval seen in other types of secondary AS, with some cases occurring within as few as 3 years. Boice [14] proposed that a tumor induced by radiation therapy will not occur until the age associated with the occurrence of the tumor type in a patient who did not receive radiation, thus the relative short latency may be related to the older age of this group of patients. The prognosis of radiation-associated AS of the breast is generally poor with unpredictable clinical behavior, and there is a high rate of local and distant recurrence [13]. The 5-year-survival rate is approximately 50% [15].
AS of the breast presents clinically as erythematous plaques or nodules, with multifocality or diffuse involvement of the breast not uncommon [13]. Histologically, AS can show heterogenous features, with well-differentiated areas mixed with or directly adjacent to poorly-differentiated components. Extension into the subcutis as well as the breast parenchyma itself is frequently observed, and various combinations of patterns (vasoformative, sievelike, kaposiform, or solid) can be present within a single tumor (Figure 1) [13]. Endothelial cell multilayering is a feature, and intraluminal blood or “blood lakes” may be present [16]. Malignant cells demonstrate prominent nucleoli, brisk mitotic activity, and an infiltrative growth pattern with prominent dissection between dermal collagen bundles (Figures 2-5) [17]. The surrounding normal tissue may show radiation-induced changes including homogenized collagen and mildly atypical dermal fibroblasts [6]. When immunohistochemistry is necessary to establish the origin of the tumor cells, CD31 is the single best marker with high sensitivity and specificity, though caution in interpretation is required as it can also stain macrophages (Figure 6) [13]. Erythroblast transformation specific related gene (ERG), a nuclear stain which is equally as sensitive but not as specific as CD31, is another useful marker that can complement the cytoplasmic/membranous staining of CD31 (Figure 7) [18]. CD34 may be negative in poorly-differentiated areas (Figure 8) [19]. Aberrant cytokeratin expression is an important pitfall, especially in epithelioid AS [13,15].
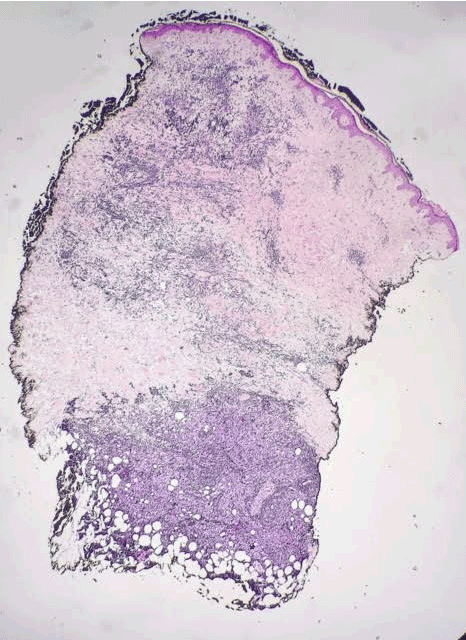
Figure 1. AS. Biopsy showing poorly-formed vessels infiltrating the subcutis. H&E, 20x.
\
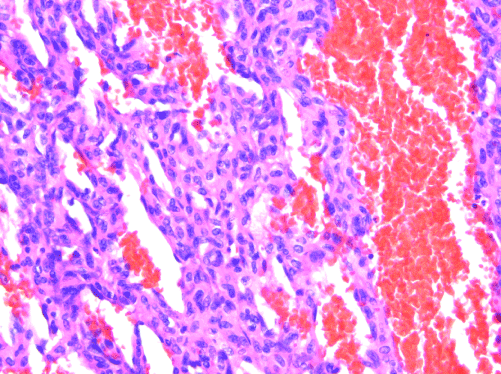
Figure 2. AS. Endothelial multilayering and “blood lakes” are common features. H&E, 200x.

Figure 3. AS. Malignant endothelial cells encircling individual collagen bundles. H&E, 200x.
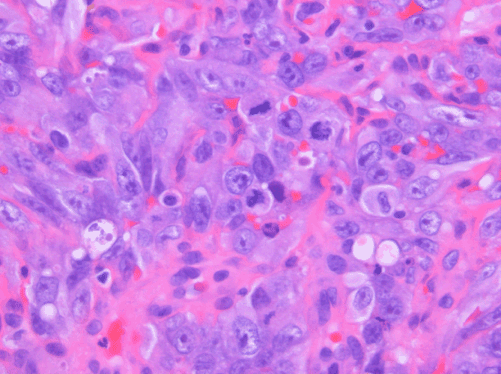
Figure 4. AS. Nuclear pleomorphism and mitotic activity are evident. H&E, 400x.
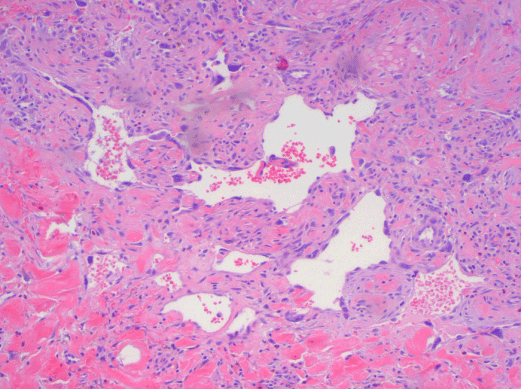
Figure 5. AS. Anastomosing vascular channels lined by atypical endothelial cells. H&E, 200x.
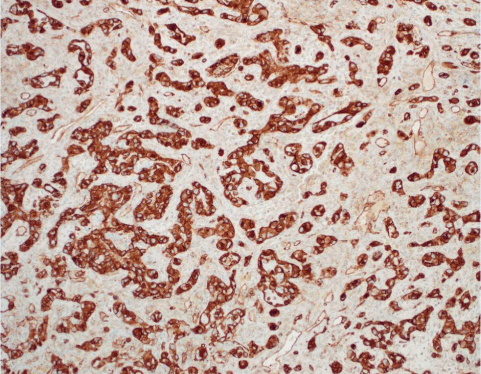
Figure 6. AS. CD31 highlights the endothelial cells. CD31 IHC.
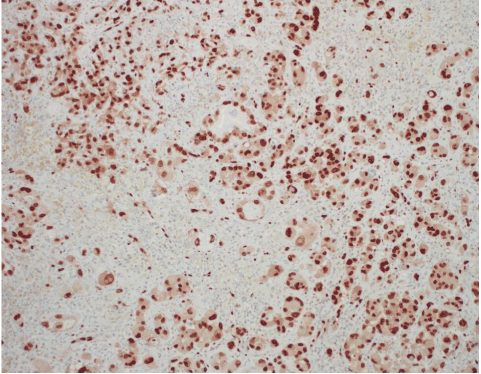
Figure 7. AS. ERG highlights the endothelial cells (nuclear stain). ERG IHC.
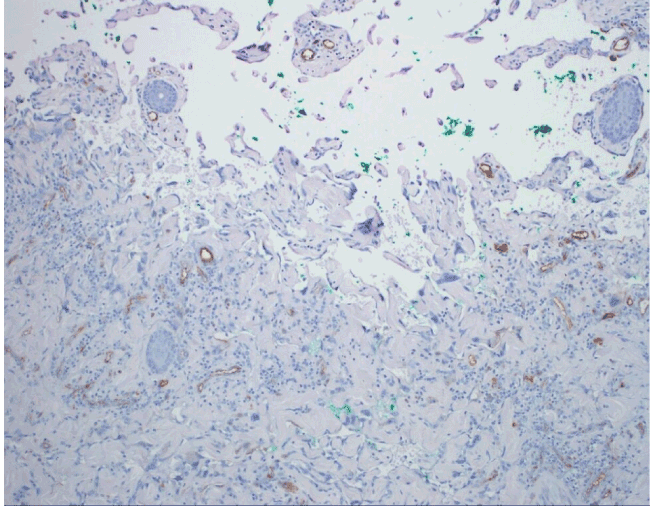
Figure 8. An example of angiosarcoma with aberrant loss of CD34 expression.
Atypical vascular lesion
In 1994, Fineberg and Rosen [17] reported 4 cases of an unusual vascular lesion occurring in mammary skin after radiation, which they named atypical vascular lesion (AVL). Their clinical behavior has so far been reported to be benign, though local recurrence as well as new, independent AVLs developing asynchronously have been described [9,16,17,20,21]. There have been rare reports of AVL progressing to angiosarcoma, which raises the question whether AVL may be a precursor lesion to AS [16,22]. AVL is a specific entity (rather than a descriptive term) and, though first described in the setting of breast-conserving therapy, it should be noted that the development of AVL is not restricted to a specific anatomic site or reason for radiation, as AVLs have also been reported on the lower extremity, flank, vulva, and abdomen [16,20,23-27].
Clinically, AVL presents as single or multiple small, flesh-colored papules or erythematous patches. Though there may occasionally be overlap of clinical features with AS, in general, the clinical appearance can be quite useful in making this distinction. Latency interval from radiation therapy to time of diagnosis is similar to that of secondary AS. Histologically, AVL is a small, delimited lesion which is confined to the dermis (Figure 9). It has vascular channels that infiltrate between collagen bundles (Figure 10). It may have hobnail and hyperchromatic endothelial cells, but it lacks the marked nuclear atypia that is usually seen in AS (Figure 11). Mitoses, necrosis, and “blood lakes” are absent, and vascular channels may be more dilated at the superficial aspect of the lesion, becoming more compressed in the deeper reaches of the dermis [16]. A patchy chronic inflammatory infiltrate is often present. Two histologic patterns have been described, the lymphatic type (LT) resembling lymphangioma, and the vascular type (VT) consisting of slit-like vascular spaces lined by hobnail endothelium [22]. Most AVL’s reported in the literature are of the lymphatic type - out of the more than 80 reported cases of lymphatic type AVL, only two of these have progressed to AS [16,22]. Patton et al. [22] proposed that the risk of developing AS seems to be greater with VT AVL than with LT AVL. Given the spectrum of changes seen in AVL as well as in AS, the question remains as to whether these lesions are on a morphologic continuum and, therefore, whether AVL is a potential precursor to AS. A comparison of clinical and histologic features of AVL and AS are summarized in Tables 1 and 2.
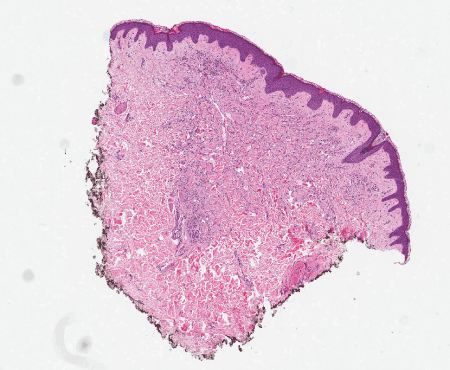
Figure 9. AVL. The lesion is small, delimited, and confined to the dermis in this punch biopsy. H&E, 20x.

Figure 10. AVL. Vascular channels infiltrate between collagen bundles. H&E, 100x.
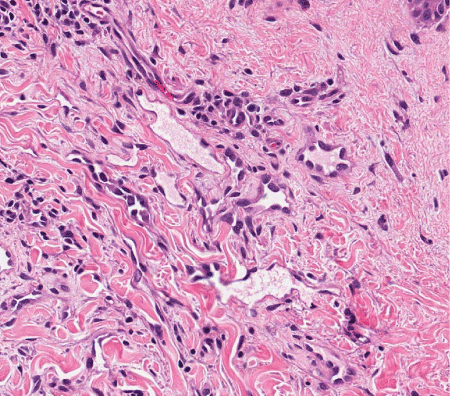
Figure 11. AVL. The endothelial cells show hobnailing and hyperchromasia, but significant nuclear atypia is absent. H&E, 200x.
Study |
AVL |
sAS |
sAS
non-breast site |
Age |
Latency (yrs) |
Size of Lesion |
Age |
Latency (yrs) |
Size of Lesion |
Latency
(yrs) |
Fineberg and Rosen
|
68 |
3.1 |
|
72 |
4.1 |
|
|
Billings et al.
|
N/A |
N/A |
|
69 |
4.9 |
|
|
Patton et al.
|
61 |
6 |
0.8 cm |
N/A |
N/A |
|
|
Fernandez et al. |
54 |
6.5 |
|
74 |
7.2 |
|
|
Brenn and Flether |
57 |
3.5 |
0.5 cm |
68 |
5.5 |
7.5 cm |
30 |
Chen et al. |
|
|
|
|
|
|
17 |
Wiklund et al. |
|
|
|
|
|
|
13 |
Ginter et al. |
67.3 |
5.3 |
|
72.7 |
7.8 |
|
|
Table 1. Age at diagnosis of vascular lesion, clinical size, and latency interval for AVL, secondary mammary AS, and secondary AS from non-breast sites
Abbreviations: AVL= atypical vascular lesion; sAS= secondary angiosarcoma.
Latency interval refers to elapsed time between radiation therapy and diagnosis of vascular lesion.
(Fineberg and Rosen, 1994, Billings et al., 2004, Patton et al., 2008, Fernandez et al., 2012, Brenn and Fletcher, 2005, Chen et al., 1979, Wiklund et al., 1991, Ginter et al., 2014)
Table 2. Histologic features of AVL and AS
|
AVL |
AS |
Relative circumscription |
+++ |
- |
Intraluminal projections |
+ |
- |
Associated chronic inflammation |
++ |
++ |
Hyperchromatic and hobnail endothelial cells |
++ |
+++ |
Complex dissecting growth pattern |
+ |
+++ |
Necrosis |
- |
+ |
“Blood lakes” |
- |
++ |
Infiltration into subcutis |
- |
++ |
Significant nuclear atypia |
- |
+++ |
Endothelial multilayering |
- |
+++ |
Mitoses |
- |
+++ |
From Fineberg and Rosen and Brenn and Fletcher. (Brenn and Fletcher, 2005, Fineberg and Rosen, 1994)
Abbreviations: AVL= atypical vascular lesion; AS = angiosarcoma; - = absent, + = occasionally present; ++ = present in most cases; +++ = present in all cases
Advances in ancillary testing: MYC amplification and anti-MYC immunohistochemistry
Given the clinical consequences of a correct diagnosis of AS and the significant overlap of histologic features which can be encountered on small biopsies, a clear need exists for ancillary testing to aid in this important distinction.
In other diagnostically difficult scenarios in pathology, immunohistochemistry (IHC) is often utilized to help discern between morphologically similar entities. AVL has been proposed to represent a lymphatic lesion; however, positive staining with the monoclonal antibody D2-40, a selective marker of lymphatic endothelium, has been observed in both AVL and AS, thus limiting the utility of this marker in this scenario [20,28-30]. The utility of other IHC to resolve this particular dilemma has not otherwise been pursued with the exception of rare studies [31].
In 2010, Manner et al. [32] used array-comparative genomic hybridization and found high level amplification of chromosome 8q24.21 (corresponding to the gene MYC) exclusively in 55% of AS secondary to irradiation and chronic lymphedema, but not in primary AS. Studied cases included breast and non-breast sites. This study proposed that primary and secondary AS represent genetically distinct entities, though more recent studies have described examples of MYC amplification in primary cutaneous AS from various sites [33,34]. A later study further investigated the oncogenic role of MYC to include radiation-induced AVL, finding MYC amplification by fluorescence in situ hybridization (FISH) in 100% of secondary mammary AS and none in AVL, primary AS at any site, or in secondary sarcomas other than AS [29]. Importantly, when looking at excision specimens for AS, this study found MYC amplification only in the AS cells; no MYC abnormality was detected in AVL lesions seen adjacent to AS within the same mastectomy specimen. Fernandez and others reinforced these findings, showing MYC amplification by FISH in all cases of secondary AS but not in AVL [35]. Application of anti-MYC IHC showed strong positive nuclear staining in all cases of AS but none in AVL. Ginter et al. [33] found 100% concordance between MYC amplification and protein expression in AVL (negative), primary mammary AS (negative), and secondary mammary AS (positive). The staining pattern was moderate-strong and diffuse (>80% of neoplastic cells) in all positive cases, whereas complete absence of staining was seen in negative cases. When interpreting anti-MYC IHC, intervening benign capillary vessels should not be mistaken for non-immunoreactive neoplastic vessels, and it should be noted that lymphocytes can be positive for anti-MYC and may be problematic if they are so numerous as to obscure the vessels of interest. FISH for MYC amplification has a specificity of 100% and a sensitivity of approximately 80%, indicating that a negative result does not fully exclude a diagnosis of AS [35]. This is likely due to sampling or technical errors. FISH studies and anti-MYC IHC for AVL and AS are illustrated in Figures 12–19. Table 3 summarizes the findings of the studies to date regarding MYC amplification in primary mammary AS, secondary mammary AS, and AVL.

Figure 12. AS. H&E, 144x.
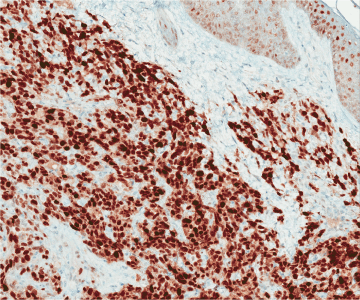
Figure 13. Corresponding anti-MYC IHC. Nuclear staining is strong and diffuse. MYC IHC, 148x.
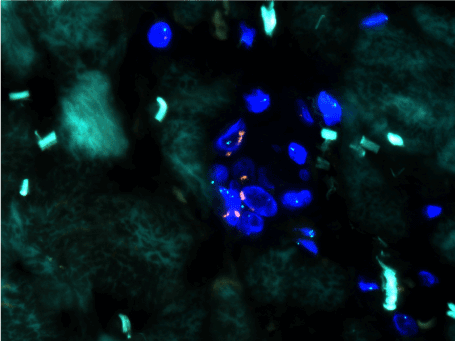
Figure 14. Positive FISH for MYC (red signal) amplification.
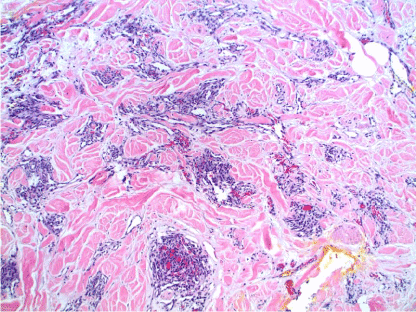
Figure 15. Well-differentiated AS. Well-formed, slit-like vascular spaces lined by hyperchromatic nuclei dissect the dermal collagen. There is associated chronic inflammation. H&E, 100x.
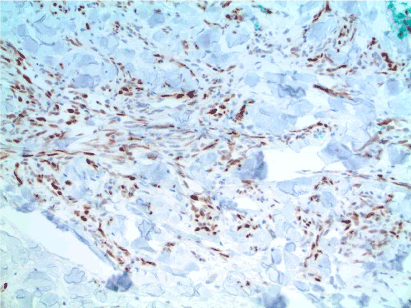
Figure 16. Well-differentiated AS. Strong, positive nuclear staining of the atypical endothelial cells supports the diagnosis. MYC IHC, 100x. Case and image courtesy of Greg Hosler, MD, PhD.
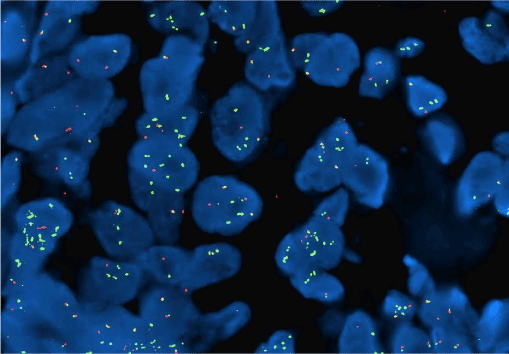
Figure 17. Well-differentiated AS. Using a MYC break apart FISH probe, a portion of the MYC gene is amplified (green).
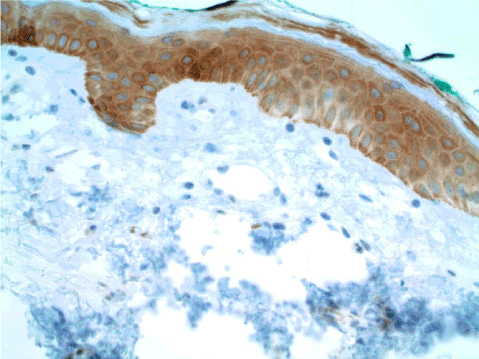
Figure 18. AVL. The endothelial cells are negative. MYC IHC, 100x.
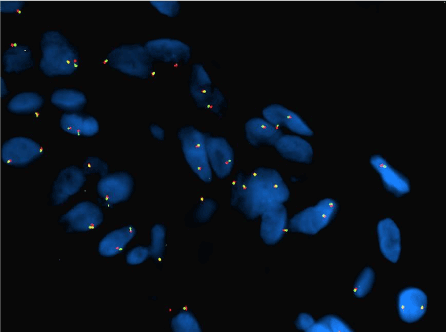
Figure 19. AVL. Using a MYC break apart FISH probe, no amplification is identified.
Table 3. Summary of FISH and IHC studies to date for primary mammary AS, secondary mammary AS, and AVL
|
Interphase FISH for
MYC Amplification |
Anti-MYC IHC |
FISH/IHC Concordance (%) |
Study |
pMAS |
sMAS |
AVL |
pMAS |
sMAS |
AVL |
pMAS |
sMAS |
AVL |
Manner et al |
0/2 |
Data not available* |
|
|
|
|
|
|
|
Guo et al |
0/6 |
22/22 |
0 /12 |
|
|
|
|
|
|
Mentzel et al |
0/8 |
25/25 |
0/13 |
0/2 |
21/22 |
1/16† |
100 (2/2) |
95 (21/22) |
92 (12/13) |
Fernandez
et al |
|
6/6 patients
6/8 specimens |
0/3 |
|
6/6 patients
7/8 specimens |
0/3 |
|
100
(6/6)
88
(7/8) |
100
(3/3) |
Ginter et al |
0/17 |
9/9 |
0/7 |
0/17 |
9/9 |
0/7 |
100 (17/17) |
100 (9/9) |
100
(7/7) |
Abbreviations: pMAS = primary mammary angiosarcoma; sMAS = secondary mammary angiosarcoma; AVL = atypical vascular lesion
*MYC amplification was found in 18/33 (55%) cases of secondary AS, including 16 radiation-induced AS from all sites and 2 AS associated with chronic lymphedema
†One case showed focal positivity
(Manner et al., 2010, Guo et al., 2011, Mentzel et al., 2012, Fernandez et al., 2012, Ginter et al., 2014)
Conclusion
Secondary mammary AS, although rare, accounts for up to 40% of all radiation-induced sarcomas [36]. With continued treatment of breast cancer with breast-conserving surgery and adjuvant radiation, dermatopathologists are likely to increasingly encounter biopsies of vascular lesions of the breast occurring in this setting. The primary differential diagnosis is secondary AS and AVL, a distinction which can be made with relative ease in large excision specimens but can be rather challenging on initial biopsy. There can be considerable clinical and histologic overlap between radiation-associated AVL and cutaneous AS, making this important distinction difficult at times. The inherent histologic heterogeneity of AS, with “AVL-like” areas of AS not uncommon especially near the tumor periphery, cause sampling bias to add to the difficulty in exact classification [11,16,35].
Manner et al. proposed that secondary AS is driven by MYC alterations and is distinctly different from primary AS. Subsequent studies have confirmed MYC amplification in secondary AS, and found lack of amplification in radiation-induced AVL [29,32,33,35,37]. Anti-MYC IHC has been shown to be a sensitive marker in AS, and could serve as a useful diagnostic tool for pathologists as IHC is more widely available and less costly than FISH. In addition, IHC may be helpful for mapping these lesions and for exact control of tumor-free margins [37].
Currently, these diagnostic tools are not widely available to all dermatopathologists. When confronted with one of these difficult vascular lesions on the breast, a simple phone call to the clinician or surgeon can be extraordinarily helpful, especially if little clinical information has been provided. If there is obvious discordance between the clinical impression and the histologic findings, then likely a sampling issue is the culprit and repeat biopsies would be helpful. If, on the other hand, both the clinical and histologic impression are equivocal, pursuing ancillary studies to make a correct diagnosis is prudent.
Rare reports of AVL progressing to AS raise the question as to whether AVL and AS are on a morphologic continuum, with AVL representing a precursor lesion, or whether these lesions are distinct biologic entities. Given the lack of MYC amplification in AVL, their role as true precursor lesions could be questioned; however, it has been shown convincingly, though in rare cases, that progression to AS has occurred. As such, complete excision of post-radiation atypical vascular lesions is prudent if clinically feasible, and close clinical follow-up is strongly recommended [37].
Acknowledgements
I would like to thank the following for their generous contributions to this article:
Steven D. Billings, MD
Co-Director Dermatopathology Section
Department of Pathology
Cleveland Clinic
9500 Euclid Ave. L25
Cleveland, OH
Gregory A. Hosler, MD, PhD
Dermatopathologist
ProPath Dermatopathology
1355 River Bend Dr.
Dallas, TX 75247
Nathan Lee, MD
Dermatopathology Fellow, Department of Pathology
University of Arkansas for Medical Sciences
4301 W. Markham St., Mail slot 517
Little Rock, AR 72205
References
- Mattoch IW, Robbins JB, Kempson RL, Kohler S (2007) Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol 57: 126-133. [Crossref]
- Stewart FW, Treves N (1948) Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer 1: 64-81. [Crossref]
- Cabioglu N, Hunt KK, Buchholz TA, Mirza N, Singletary SE, et al. (2005) Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer 104: 20-29. [Crossref]
- Pass H, Vicini FA, Kestin LL, Goldstein NS, Decker D, et al. (2004) Changes in management techniques and patterns of disease recurrence over time in patients with breast carcinoma treated with breast-conserving therapy at a single institution. Cancer 101: 713-720. [Crossref]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, et al. (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366: 2087-106. [Crossref]
- Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW (2004) Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol 28: 781-788. [Crossref]
- Strobbe LJ, Peterse HL, van Tinteren H, Wijnmaalen A, Rutgers EJ (1998) Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseen sequela. Breast Cancer Res Treat 47: 101-109. [Crossref]
- Fodor J, Orosz Z, Szabó E, Sulyok Z, Polgar C, et al. (2006) Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol 54: 499-504. [Crossref]
2021 Copyright OAT. All rights reserv
- Monroe AT, Feigenberg SJ, Mendenhall NP (2003) Angiosarcoma after breast-conserving therapy. Cancer 97: 1832-1840. [Crossref]
- Hodgson NC, Bowen-Wells C, Moffat F, Franceschi D, Avisar E (2007) Angiosarcomas of the breast: a review of 70 cases. Am J Clin Oncol 30: 570-573. [Crossref]
- West JG, Qureshi A, West JE, Chacon M, Sutherland ML, et al. (2005) Risk of angiosarcoma following breast conservation: a clinical alert. Breast J 11: 115-123. [Crossref]
- Kiluk JV, Yeh KA (2005) Primary angiosarcoma of the breast. Breast J 11: 517-518. [Crossref]
- Lucas DR1 (2009) Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med 133: 1804-1809. [Crossref]
- Boice JD (1981) Cancer following medical irradiation. Cancer 47: 1081-1090. [Crossref]
- Meis-Kindblom JM, Kindblom LG (1998) Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 22: 683-697. [Crossref]
- Brenn T, Fletcher CD (2005) Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol 29: 983-996. [Crossref]
- Fineberg S, Rosen PP (1994) Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol 102: 757-763. [Crossref]
- Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, et al. (2015) The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol 68: 44-50. [Crossref]
- Poblet E, Gonzalez-Palacios F, Jimenez FJ (1996) Different immunoreactivity of endothelial markers in well and poorly differentiated areas of angiosarcomas. Virchows Arch 428: 217-221. [Crossref]
- Diaz-Cascajo C, Borghi S, Weyers W, Retzlaff H, Requena L, et al. (1999) Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology 35: 319-327. [Crossref]
- Gengler C, Coindre JM, Leroux A, Trassard M, Ranchare-Vince D, et al. (2007) Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process: a study from the French Sarcoma Group. Cancer 109: 1584-1598. [Crossref]
- Patton KT, Deyrup AT, Weiss SW (2008) Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol 32 943-950. [Crossref]
- Ambrojo P, Cogolludo EF, Aguilar A, Sunchez Yus E, Sunchez de Paz F (1990) Cutaneous lymphangiectases after therapy for carcinoma of the cervix--a case with unusual clinical and histological features. Clin Exp Dermatol 15: 57-59. [Crossref]
- Fisher I, Orkin M (1970) Acquired lymphangioma (lymphangiectasis). Report of a case. Arch Dermatol 101: 230-234. [Crossref]
- Jappe U, Zimmermann T, Kahle B, Petzoldt D (2002) Lymphangioma circumscriptum of the vulva following surgical and radiological therapy of cervical cancer. Sex Transm Dis 29: 533-535. [Crossref]
- Schwab RA, McCollough ML (2001) Acquired vulvar lymphangiomas: a sequela of radiation therapy. Cutis 67: 239-240. [Crossref]
- Weyers W, Nilles M, Kanig M (1990) [Lymphangioma circumscriptum cysticum following surgical and radiologic therapy]. Hautarzt 41: 102-104. [Crossref]
- Kahn HJ, Bailey D, Marks A (2002) Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 15: 434-440. [Crossref]
- Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR (2011) Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 50: 25-33. [Crossref]
- Requena L, Kutzner H, Mentzel T, Durun R, Rodraguez-Peralto JL (2002) Benign vascular proliferations in irradiated skin. Am J Surg Pathol 26: 328-337. [Crossref]
- Shin SJ, Lesser M, Rosen PP (2007) Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med 131: 538-544. [Crossref]
- Manner J, Radlwimmer B, Hohenberger P, Massinger K, Kuffer S, et al. (2010) MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 176: 34-39. [Crossref]
- Ginter PS, Mosquera JM, MacDonald TY, D'Alfonso TM3, Rubin MA, et al. (2014) Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol 45: 709-716. [Crossref]
- Shon W, Sukov WR, Jenkins SM, Folpe AL (2014) MYC amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in-situ hybridization and immunohistochemical study. Mod Pathol 27: 509-515. [Crossref]
- Fernandez AP, Sun Y, Tubbs RR, Goldblum JR, Billings SD (2012) FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol 39: 234-242. [Crossref]
- Lagrange JL, Ramaioli A, Chateau MC, Marchal C, Resbeut M, et al. (2000) Sarcoma after radiation therapy: retrospective multiinstitutional study of 80 histologically confirmed cases. Radiation Therapist and Pathologist Groups of the Federation Nationale des Centres de Lutte Contre le Cancer. Radiology 216: 197-205. [Crossref]
- Mentzel T, Schildhaus HU, Palmedo G, Battner R, Kutzner H (2012) Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol 25: 75-85. [Crossref]



















