We contrived a color-imaging manner in histodiagnosis of cancer and evaluated its clinical feasibility and significance. Cancer was microscopically detected by identifying specific reddish pink fluorescence emitted from cancer cells under blue light irradiation, which was based on a photodynamic diagnosis (PDD) theory. Aminolevulinic acid (ALA) was used to treat the histologic samples. Three different types of tumor cell lines were used as neoplastic models. In in vitro, we ascertained that each tumor cell treated with ALA-contained medium solution emitted red fluorescence under blue light. In clinical studies, freshly harvested specimens from cancer patients during surgical operations were directly soaked into ALA-containing culture medium,, which were incubated at 37℃ for certain minutes. Then microscopically observed under the blue light beam. The samples radiated reddish pink fluorescence were regarded as cancer. Each model cell emitted pink fluorescence by treating with 1% ALA/cell culture medium at 37℃ for 30 min. Non-viable and/or apoptotic cells showed no fluorescence under the same conditions. Clinically harvested cancer regions treated with the ALA-medium solution also showed pink fluorescence. We could discriminate malignant change of the samples within 30-60 minutes without preparing frozen sections for rapid diagnoses. This simple method suggested clinically useful tool in a rapid cancer diagnosis.
histodiagnosis, reddish pink fluorescence, aminolevulinic acid, rapid cancer diagnosis
Aminolevulinic acid (ALA) is an endogenous amino acid. The ALA incorporated into cells is synthesized into protoporphirin IX (PPIX). In cancer cells, PPIX synthesis is significantly enhanced, while the PPIX metabolizing pathway is inhibited. The PPIX consequently accumulates in cancer cells. PPIX emits red fluorescence when irradiated with a blue light. On the basis of this phenomenon, a photodynamic diagnosis (PDD) has been clinically performed [1,2]. According to this rationale, we carried out a novel photodynamic histodiagnosis (PDHD) using our simple tools in the laboratory.
A visible blue light: SOLARFORCE, L2P, Hong Kong. Aminolevulinic acid (ALA): Wako Pure Chemical Industries, Ltd. Osaka, Japan. HCT 116 cells and HeLa cells: RIKEN BRC Cell Bank, Tsukuba, Japan. HFSKF-II cells: Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan. RPMI 1640 cell culture medium (RPMI): Life Technologies Corp, Grand Island, NY, USA. Ham F-12 medium (Ham F-12): Gibco by life technologies, USA. Fetal bovine serum (FBS): Gibco, by life technologies, USA. Other reagents used were analytical grade.
First, we confirmed the ALA-PDD phenomenon in vitro. 1 x 104 counts of model cells were individually cultured in T 12.5 plastic flasks. After reaching to confluent, the medium in the flask was wholly replaced to 1% ALA-containing medium to re-culture for 24 hours moreover. Then, each was microscopically examined under the blue light in a dark room to observe the reddish-pink fluorescence. The time we could clearly recognize the red fluorescence was periodically measured at 0, 15, 30 and 60 minutes, after starting the re-incubation.
Next, we tried clinical histodiagnosis on the specimens surgically removed from patients with colorectal cancer. The samples freshly harvested were at once immersed into 1% ALA/RPMI medium solution to incubate for 30 min. at 37°C, then, such treated specimens were microscopically examined under the blue light in a dark room. Normal portions in the same surgical specimen were served as the controls.
The model cells cultured in vitro clearly emitted reddish pink fluorescence by the 1% ALA/medium solution, at 37℃ within 30-60 min (Figure1). Non-viable and/or apoptotic cells showed no fluorescence.
Cancer specimens harvested during operation also emitted the similar fluorescence by treating in the same way, and we could easily identify them as cancer by observing with a magnifying glass (Figure 2). Those treated without ALA solution showed no red fluorescence (Figure 3). Our ALA-PDHD results coincided with those routine pathological examinations with Hematoxylin and eosin (H&E) stain.
2021 Copyright OAT. All rights reserv
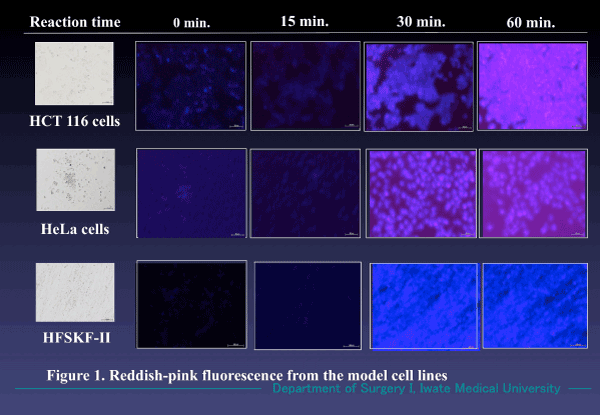
Figure 1. Fluorescence emitted in the model cell lines. Reddish pink fluorescence appeared 30 min. after incubation in a 1% ALA/RPMI medium solution. Not only cancer cells (HCT 116 and HeLa) but also multiplying benign cells (HFSKF-II) also showed similar florescence. (Magnification X 100).
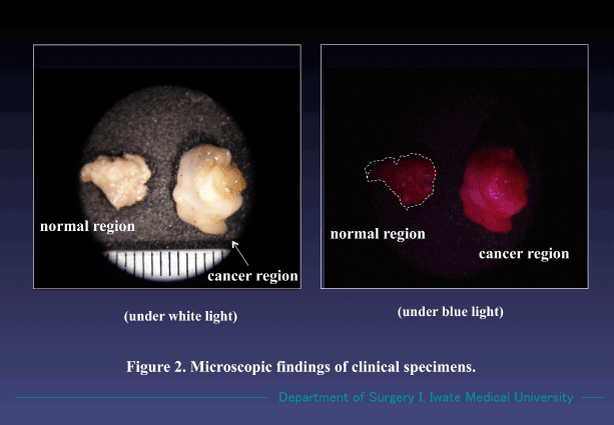
Figure 2. Findings of cancer and non-cancer regions were treated with 1% ALA/RPMI mixed solution for 30 min. The cancer region specifically emitted red fluorescence under the blue light. Non-cancer region showed no fluorescence. Both samples radiated no fluorescence under white light. (Magnification X 10).
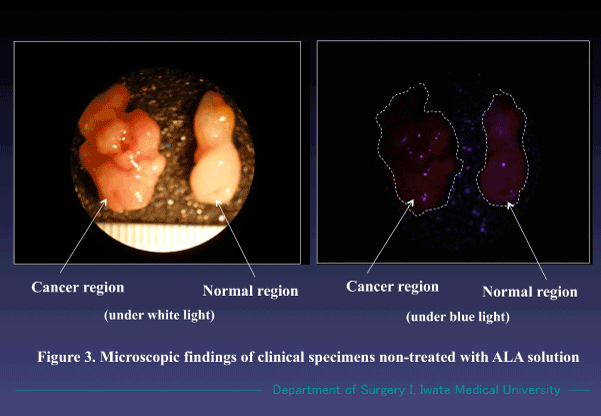
Figure 3. Findings of cancer and non-cancer regions treated with RPMI medium solution alone for 30 min. No fluorescence was observed under the blue light. (Magnification X 10).
Our novel color-imaging method suggested clinically effective modality for a rapid diagnosis. We could diagnose cancer or non-cancer of the intraoperative specimens within 30-60 minutes after the harvest.
Cancer or non-cancer could be identified by this novel approach, while, discrimination of pathologic types of the specimens was impossible with this technique. Accordingly, we consider that this is useful as supporting tool of the routine rapid diagnoses with frozen sections and particularly worth-while in an intraoperative detection of metastatic lymph nodes in cancer patients to conduct minimally invasive surgery.
In preparation of histologic samples for this ALA-diagnosis, the specimens should be viable, since biological metabolizing pathway from ALA to protoporphyrin IX (PPIX) is absolutely necessary for this photo-chemical diagnosis. Surgically harvested specimens, therefore, should be treated with ALA-medium solution in a short time. To maintain the cell viability of the surgical specimens, we used RPMI 1640 medium with 10% FBS for cell culture solution, in which we added 1% ALA/physiological saline solution as a photosensitizer at the volume ratio of one to one. The pH value of such prepared mixture showed pH 5. The ALA solution at pH near 5 is reported to be most stable, and at pH values higher than 7, it becomes instable [3]. A 0.1-2% ALA in sodium chloride solution with pH 5 was recommended for a clinical use [4]. Since our own 1% ALA-RPMI mixed solution was at pH 5, it was appropriate for preparation of the treatments for this method.
In this connection, we compared intensity of the fluorescence emitted from the clinical specimens which had been treated with the 1% ALA/RPMI solution and treated with 1% ALA/physiological saline solution at the same time. No significant difference of the fluorescent intensity was observed between the two in the cases of 30 minutes’ incubation. We considered that RPMI solution used for PDHD preparation had almost no influence on appearance of the fluorescence for 30 minutes’ incubation. While in the cases of 60 minutes’ incubations, that of treated with the ALA/RPMI solution showed far clearer red fluorescence than that of treated with ALA solution. Since the strength of the fluorescence represented the viability of the cells, postoperative viability of tissues harvested during surgery were considered to be more stably maintained by treating with the RPMI solution than treating with ALA solution alone. The RPMI-containing medium solution suggested playing an important role in preserving the cell viability. On these data, we would like to propose that the intraoperative specimens ought to be treated with RPMI-contained ALA solution within 30-60 min. for PDHD method.
In a routine intraoperative rapid diagnosis, frozen sections of the clinical specimens are prepared. To make ready for such histologically detectable sections, usually it takes 30-60 minutes. Additionally, experienced pathologists are always asked to precisely diagnose with the frozen sections to evade misdiagnoses following outbreak of degeneration of the materials due to inadequate treatments during intricate preparation process.
In PDHD, only the detection of red fluorescence enables us to find out cancer lesions. We consider histo-pathological experiences are not always necessary for diagnoses with the PDHD manner.
We could practically detect a bile tract cancer with this method. In this case, the precipitate harvested from less than 10 mL of bile obtained via a PTCD tube was treated with 3 mL of 1% ALA-RPMI mixed solution for 30 min, according to our routine method. Such treated material was centrifuged to prepare a smear sample, which was microscopically observed under the blue light. We could find a reddish-pink fluorescence in such treated section and preoperatively enabled to diagnose as bile tract cancer [5]. The section was also treated in Papanicolaou stain which was found to be a cancer. By the routine cytological examination, this case had been diagnosed as an inflammation at the bile tract with atypically degenerated cells. No further pathological findings were indicated with the routine smear sections.
The procedures conducted in this case may be called as a photodynamic cytodiagnosis (PDCD). The PDCD is also promising for rapid diagnosis of cancer in the ascites and /or pleural effusion. Both PDHD and PDCD warrant using ALA and RPMI-medium solutions further studies in clinical rapid diagnoses.
- Navone NM, Polo CF, Frisardi AL, Andrade NE, Battle AM (1990) Heme biosynthesis in human breast cancer-mimetic “in vitro” studies and some heme enzymic activity levels. Int J Biochem 22: 1407-1411. [Crossref]
- Baumgartner R, Fisslinger H, Jocham D, Lenz H, Ruprecht L, et al. (1987) A fluorescence imaging device for endoscopic detection of early stage cancer-instrumental and experimental studies. Photochem Photobiol 46: 759-763. [Crossref]
- Gadmar OB, Moan J, Scheie E, et al. (2002) The stability of 5-aminolevulinic acid in solution. J Photochem Photobiol B 67: 187-193. [Crossref]
- De Blois AW, Grouls RJ, Acherman EW, Wijdeven WJ (2002) Development of a stable solution od 5-aminolevulinic acid for intracuteneous injection in photodynamic therapy. Lasers Med Sci 17: 208-215. [Crossref]
- Ito N, Sugitachi A, Takahashi M, Makabe K, Kanno S, et al. (2013) Photodynamic diagnosis and preoperative chemotherapy for biliary tract cancer. Jpn J Cancer Chemother 40: 1641-1643. [Crossref]



