Abstract
Brain and Cognitive reserve concept is a theoretical general framework introduced to explain individual differences to withstand brain damages. Several behavioural and neuroimaging studies support the evidence that lifestyles act on brain plasticity modulating the impact of neurological insults. However, once that AD is clinically evident, the protective benefits of premorbid experience are assumed to be substantially decreased. This review presents an overview of the principal literature focused on reserve mechanisms. The evolution of the conceptual framework is considered, passing from brain and cognitive to neural reserve’s hypothesis. Interactions between reserve mechanisms and AD-related biomarkers are reported. In particular this review is focussed on the neuroimaging (MRI) studies. MRI provides the opportunity to investigate in vivo structural and functional brain changes. Several studies reported here assessed the impact of reserves on brain resilience. Literature supports the hypothesis that reserves impact on neurodegenerative process in the early phase of AD. Moreover, studies fit with the existence of a “neural reserve”, characterised by specific neural networks and their efficiency. It remains to be demonstrated whether interventions later in life can modulate this brain resilience.
Key words
brain reserve, cognitive reserve, neural reserve, proxy measure, Alzheimer’s disease, neuroimaging
Introduction
Alzheimer’s disease (AD) is the most common cause of cognitive decline in west world’s population [1]. It is a neurodegenerative disorder that typically presents with an isolated memory deficit, followed by a progressive accumulation of cognitive disabilities until conversion to dementia [1]. However, it is becoming increasingly clearer that there is a non-linear relationship between the extent of brain tissue damage and the resulting patients’ clinical outcome [2,3].
To account for these apparent inconsistencies between brain tissue damage and clinical manifestations, it has been introduced the concept of “reserve” [2,3]. The concept of a reserve’s capacity of the brain appeared for the first time in 1940, when unknown Authors stated that reserve’s tissue was highly available in many organs of body including the brain [4]. According with this view, pathological lesions in the brain resulted in an extensive destruction of nervous tissue with a little derangement of function [4]. In the last thirty years reserve’s concept has experienced several modifications.
In the traditional framework two different mechanisms were postulated for reserves: the brain reserve (BR) and the cognitive reserve (CR). BR referred to differences in brain size and other quantitative aspects of the brain (e.g. amount of neurons or synapses) that explain differential susceptibility to functional impairment in presence of pathology or neurological insult [5]. This concept arose by the observation that prevalence of dementia is lower in individuals with larger brains [6-8]. The BR implied a passive model of reserve, suggesting that the brain tolerates more accumulation of neuropathology before it reaches a critical threshold for the appearance of clinical symptoms [5]. Nevertheless, the CR implied the differences in cognitive processes as a function of lifetime intellectual activities and other environmental factors that explain the non-linear relationship between the severity of patients’ brain damage and the correspondent clinical symptoms. The CR suggested that the brain actively copes with brain damage by using the pre-existing cognitive processes or by enlisting compensatory mechanisms [3,9]. The CR model hypothesised that cognitive processes are crucial for explaining individual’ differences despite equal brain changes or pathology [10]. Cognitive processes consist of differences in cognitive efficiency, capacity or flexibility resulting from life experiences.
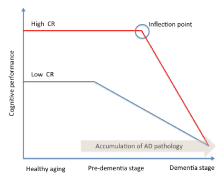
Stern and co-workers [11] addressed the concept of CR in a study on AD incidence. Analysing data from a large cohort of non-demented individuals, the Authors assumed that AD pathology slowly develops over time independently of CR, and that the pathology begins many years before the onset of clinically diagnosed AD. Individuals with greater reserves should be able to tolerate AD pathology better than those with lower CR levels. As a consequence, AD onset should be delayed in subjects with high reserve. However, in subjects with high CR cognitive decline is faster after the inflection point (Figure 1). A neuropathological study showed that CR is more able than BR to withstand neurodegeneration [8]. Indeed, individuals with high CR (as measured by education) and low BR (as measured by head size) developed dementia less than subjects with low CR and low BR [8]. During the last years, emerged that the CR relied on current neural activity to explain functional differences, suggesting that neuronal activity is modelled by cognitive, social and physical activities experienced during life. This conceptualization introduced a new way to concept the reserve’s mechanisms, based on the integrity of functional brain networks, rather than on the brain structure. Therefore, more recently, together with the CR, the “neural reserve” concept has been proposed [5]. Neural reserve involves cognitive networks’ efficiency and it provides the neural basis of CR. Individual differences in networks’ efficiency, or the use of alternative strategies, may provide reserve against the impact of brain damage. The introduction of the neural reserve concept reduces the distance between BR and CR hypotheses, and it makes differences more nuanced [5].
Static and dynamic reserve’s indexes
Reserves can be built during life through enrichment experiences [5]. These experiences regard all aspects of the individual life. Stern hypothesised that environmental factors, such as years of formal education, occupational attainment and leisure activities, may be considered as proxy measures of CR [5]. Also cognitive performances, such as the intelligence quotient (IQ) [12], memory performances, executive functioning, have been considered as proxy measures of CR [5]. More recently, reserve indexes have been categorised as static and dynamic proxy measure [13-16]. Years of formal education, occupational attainment, pre-morbid IQ and leisure activities pursued during childhood-adolescence and early adulthood life period, have been considered static measures, because they are not directly able to capture the individual cognitive changes. Conversely, memory performances and executive functioning have been considered as dynamic measure because they reflect directly cognitive measure [13-16].
However, among all static CR measures, education is considered the most relevant factor impacting on brain resilience [5,17]. Indeed, it has been stated that education may influence the development of AD in several ways. Education may induce an increase of synaptic density in the neocortex, causing a delay in the AD onset [18]. Obviously, the deterioration occurs independently of education, but the educational level may reflect cognitive capacity that allows delaying the clinical symptoms of AD, which does not become evident until the deterioration reaches a more severe degree. As a consequence, more AD pathology is needed to cause dementia in patients with higher educational level [5] (Figure 1). In addition, education could also be considered as a socializing process, promoting learning strategies, encouraging the development of divergent thinking and enabling the individual to perform more competently on cognitive demand [19]. Moreover, education appears to be an important environmental experience that may enhance neural connectivity as well as the propensity to engage in mentally stimulating activities throughout life [20]. Finally, education may improve the intellectual approach to life events [21], which can lead to lifelong mental stimulation and an enhanced activation of the brain regions involved in cognitive processing [21].
Several studies showed a decreased risk of developing AD in individuals employed in highly mentally demanding occupation [11,22,23]. Conversely, individuals without a lifetime occupation showed an increased risk of AD [24]. Evans and co-workers [22] showed that subjects with lower socioeconomic status, computed considering education, occupational attainment and income, had a higher risk of developing AD. Also Stern et al. [11] suggested that the risk of developing AD was stronger in subjects with both low education and low lifetime attainment, supporting a synergic effect of education and occupation.
Previous studies reported that high level of leisure activities performed during life whether cognitive, social and also physical; reduce the risk of developing dementia [5,25-27]. The following leisure activities are considered “reserve-builders”:
1) Cognitive activities, such as reading books, reading magazines and newspapers, producing art (e.g., painting, poetry, sculpture, song writing), producing non-artistic writing (e.g., diary, newsletter, etc.), attending lectures or organized discussions [5,25,27,28].
2) Social activities, such as playing structured games (e.g., cards, board games, crossword puzzles, etc.), participating in hobbies (e.g., gardening, model building, sewing, etc.), volunteering, entertaining friends or relatives [5,25,27,28].
3) Physical activities, such as running, trekking, swimming, biking, skiing, dancing, yoga, etc [5,25,27,28]. In addition, healthy life styles, such as Mediterranean diet, responsible drinking, limiting caffeine, nicotine and avoids big-meals, sleep hygiene, etc., are considered important reserve-enhancing factors. Several articles showed that patients with AD usually had few hobbies and were less involved in psychosocial activities during their lifetime [29-32]. Individuals engaged in frequent cognitive activities such as reading, watching television or card playing were less likely to develop AD than subjects with infrequent activities [30]. More recently, an increased risk to develop AD in amnesic MCI (a-MCI) patients with low CR level was shown, by using a comprehensive questionnaire investigating cognitive, social, physical leisure activities, education and occupation [27]. In addition, this study revealed that a-MCI patients with high CR and high baseline general cognitive efficiency (as measured by MMSE), showed a significant difference in the survival’s time compared to patients with high CR but low baseline MMSE. Specifically, patients with high CR and high baseline MMSE remained AD-free for 21 months respect to those high CR and low baseline MMSE. Conversely, no difference in the survival’s time was found in a-MCI patients with low CR and different level of baseline cognitive efficiency. Moreover, a longitudinal study in a large cohort of elderly showed that enriched cognitive life styles both reduced the risk of cognitive decline and improved the recovery of cognitive functions in the early stage of neurodegenerative diseases [33]. An association between complexity of occupation and successful aging was also found, supporting the hypothesis that more stimulating environments preserve cognitive ability later in life [34]. However, the combination of different measures of CR (such as education, occupation and cognitive lifestyle) was found more sensitive to reduce the AD risk, with respect to the different CR proxies separately [25].
However, the above-mentioned static measures have been recently considered as imprecise and few sensitive. Indeed, all these measures are not able to assess specifically the changes in patient’s cognition, and they may relate to cognitive performance for reasons other than the reserve mechanisms [13]. For instance the same level of education or of occupational attainment does not reflect the same experience in all individuals [13]. Reed and co-workers [13] proposed the concept of dynamic CR, measured in terms of changes in memory functions. Zahodne and colleagues [14,15] and Serra and co-workers [16] extended the concept of dynamic CR. These studies quantified CR as residual variance of memory scores, after accounting for demographical and brain damage variables. The residual method is in line with a definition of CR [13] as discrepancy between observed performance and expected level of performance. Therefore, individuals who perform better than predicted show positive residual score. It means that they have high CR. Conversely, subjects who perform worse than predicted show negative residual score and they have low CR. The residual variable (expressing the CR) differs from the observed score (in this case quantified as memory performance) because any variance related to demographics, brain damage and cognitive efficiency was ruled out, in addition to error. To conceptualize CR in terms of residual variance of memory functions made the CR’s concept more flexible and adaptable to cognitive changes. However, Serra and co-workers [16] have recently shown that indices of dynamic CR that strip off any contribution of general cognitive abilities to retain exclusively memory processes are not able to identify the conversion to AD efficiently. The integrity of memory function is not sufficient to withstand neurodegeneration [16]. By contrast, the ability to use synergistically the different cognitive functions is protective against the conversion to AD. Using CR measures limited to assess only memory function is likely less sensitive to detect the cognitive decline and to predict patients’ conversion [16]. According with this hypothesis dynamic CR needs a measure of general cognition to identify AD conversion efficiently. However, a dynamic concept of CR fits better than the more “static” measures based on education or lifestyle indicators with the cognitive changes due to aging and neurodegeneration.
The interaction between reserves and AD-related biomarkers
Reserve’s mechanisms cannot act independently from others biomarkers to cope the effect of AD neuropathology. Previous studies showed several significant interaction effects between levels of CR, CSF-biomarkers (β-amyloid and tau levels), genetic AD-like phenotype (APOE status), brain atrophy and the risk to develop the clinical symptoms of AD [27,35-38]. In particular, Jack and co-workers [35] showed that people with a high risk of cognitive impairment due to AD pathophysiological processes, have both APOE4 and low CR levels. On the contrary, individuals with low genetic AD risk and high CR levels better withstand AD pathology and, therefore, maintain longer normal cognitive functions. An interaction between CR, tau and phosphorylated tau (p-tau), but not β-amyloid levels, was demonstrated [37]. However, also an additive, rather than interacting, effect was reported for CR levels and AD biomarkers [39].
CSF biomarkers are increasingly used in clinical settings, but they requires invasive procedures. In parallel, quantitative neuroimaging is gaining an increasingly central role in the management and the understanding of neurodegenerative dementia. In clinical settings, Positron Emission Tomography (PET) imaging allows to detect patterns of hypometabolism suggestive for specific forms of neurodegenerative dementia [40], while ligands to specific neurobiological substrates, such as the deposition of beta-amyloid plaques [41] or intra-neuronal accumulation of tau-proteins [42], have further increased the potential of detecting specific pathological brain abnormalities in vivo.
Several neuroimaging studies have provided in vivo evidences that lifestyle acts on brain organization, confirming both BR and CR concepts. For instance, Garibotto and coworkers [43], using FDG-PET, showed positive correlation between CR measures (as education, and occupation) and cholinergic activity in the bilateral hippocampus and in the right posterior cingulate gyrus, both in AD and MCI patients. The Authors affirmed that the significant correlation found between cholinergic activity, in structures traditionally involved in memory, and CR proxies, suggests that BR in AD is associated with a preserved/stimulated cholinergic neurotransmission [43].
Recently, other studies showed a significant association between CR proxies, β-amyloid levels measured in CSF, and FDG-PET metabolism, in patients with preclinical AD [44,45]. In AD patients Aβ-carriers, higher education was associated with an altered metabolism, as measured by FDG-PET, suggesting that CR plays a compensatory function to sustain cognitive ability in presence of early AD pathology [44,45]. An association between FDG-PET metabolism in the temporoparietal cortex and high CR levels was observed also in MCI converters compared to MCI non-converters and controls [46]. Similar results were found also in prodromal AD [47].
Magnetic resonance imaging (MRI) is less expensive and non-invasive, making it an attractive alternative to nuclear medicine. In particular, quantitative MRI is able to detect non-invasively a range of tissue parameters, which are believed to reflect various pathophysiological aspects of brain damage, which may play different roles at different disease stages.
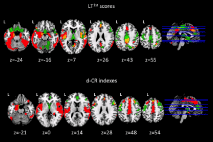
Several structural neuroimaging studies provided strong evidence of the relationship between CR levels and brain resilience. In particular, by using voxel-based morphometry (VBM), AD patients with high CR (measured as years of formal education) showed significant grey matter atrophy in the entorhinal cortex and in the temporal pole, while less educated AD patients showed widespread atrophy in the supramarginal gyrus, posterior cingulate cortex and precuneus [36]. Moreover, an association between GM volumes and reasoning abilities was found according to the CR levels. Another study [48] showed that education counteracts the effect of hippocampal atrophy, but of cerebrovascular disease as well in patients with AD. Similarly, Brickman and co-workers [49] provided evidence that healthy elderly with high CR suffered from a severe white matter damage compared with those low CR, suggesting that they were more able to cope with neurodegeneration. More recently, a significant higher risk for AD conversion was showed in a-MCI patients with higher CR levels (compared to those with low CR level) and white matter lesions in associative fasciculi [27]. Brain structural damage measured in terms of cortical thickness has been correlated to different levels of CR [50,51]. In particular, MCI patients that could convert to AD 6-12 months later, showed low CR levels and significant reduced cortical thickness at baseline, as compared to non-converter MCI [50]. More recently, Serra and co-workers [16] showed in patients with a-MCI, a different association between regional GM volumes and different measures of dynamic CR, one including memory and general cognitive efficiency scores (LT1st) and one including memory score only (d-CR). In particular, GM volumes change in the anterior (ACC) /posterior (PCC) cingulate cortex, in the left hippocampus and parahippocampal gyrus was found to be associated with all used dynamic CR measures. The common brain areas observed were likely involved both in the memory and in the more general cognitive efficiency processes [16]. Conversely, GM volumes change in the precuneus associated with the index including memory and general cognitive efficiency (LT1st), confirming the role played by the precuneus in the general cognitive abilities [16], and highlighting the presence of compensative mechanisms that allow the a-MCI patients with high CR to cope better with neurodegenerative process [16]. Moreover, the index expressing only the memory process (d-CR) associated specifically with the middle anterior cingulate cortex (pm-ACC), with the superior frontal gyrus bilaterally, with the right orbitofrontal cortex and with the right superior temporal gyrus. The pm-ACC has been found to relate with a rapid cognitive response not reflecting the correctness of the response but the cognitive activation only. Nevertheless the right orbitofrontal cortex is typically related to the go-no-go tasks and atrophy in this brain area may be lead to the inhibition deficits. The Authors hypothesised that the patients reaching positive values in the d-CR index (and consequently high CR) showed more cognitive promptness, but not necessary more accuracy in the response (as typically observed in patients with low CR). More interesting, although The Authors did not perform a statistical formal comparison of the association between GM volume and the dynamic CR scores between a-MCI and healthy elderly (HE), a qualitative analysis revealed that LT1st was mainly associated, in both groups, with the volume of PCC/precuneus and ACC. Conversely the residual score (expressing the d-CR) was mainly associated with the volume of the most anterior portion of ACC (Figure 2). These overlapping results confirm that the integrity of the cingulate cortex is crucial for cognitive efficiency.
Resting-state fMRI (RS-fMRI) allows investigating functional brain networks and assessing functional connectivity between different brain areas. Relationship between CR and brain networks, which are the closest representations we can obtain of neural reserve in vivo, have been assessed in several studies. RS-fMRI data can be analysed using different methodological approaches, both by using regions of interest- (RoI) and by using whole-brain analyses.
Bozzali and co-workers [38], by using a RoI-based RS-fMRI approach, showed that CR modulated the functional connectivity in the posterior cingulate cortex, whose disconnection with the temporal lobes is known to be critical for the conversion from MCI to AD. This effect was highly significant in AD patients, less evident in patients with MCI, and totally absent in healthy subjects [38]. The Authors hypothesised that CR effects observed in patients were primarily the result of neural compensation (i.e., compensatory resources recruited to cope with brain change) rather than neural reserve (i.e., pre-existing resources determining individual performance in the absence of pathology) [5,38].
An other promising technique is based on the whole-brain analysis driven by graph theory [52,53], a mathematical approach that describes complex systems as networks [52,53]. In simple words, the brain is conceptualized as a number of regions (nodes) that are functionally connected to each other by edges, and whose importance and efficiency within the whole network is determined by their functional specialization (i.e., segregation) and integration. In this view, some nodes are more critical (i.e., centrality) for information processing (efficiency in information transferring) and are called “hubs”. Abnormal connectivity between “hubs” is believed to cause more deficits than that between peripheral nodes [52,53]. Recently, Serra and co-workers [16], showed connectivity differences between high and low CR only in a-MCI patients, by using the graph theory [52,53]. In particular, participants with high CR showing a significant increase of connectivity in a network involving mainly fronto-parietal nodes. Conversely, they showed significantly decreased connectivity in a network involving fronto-temporo-cerebellar nodes. These findings supported the hypothesis that CR impacts on neurodegenerative process in the early phase of AD only. In addition, results fit with the existence of a “neural reserve”, characterised by specific neural networks and their efficiency.
Conclusions
Literature supports the existence of reserve mechanisms that withstand neuropathology. Brain, cognitive and more recently, neural reserve have been documented using different approaches. Interactions between reserve and several biomarkers have been proved. Therefore, it is becoming clearer that healthy lifestyles impact on brain networks. Cognitive functioning and its efficiency in the presence of AD pathology reflect the efficiency of these networks. It remains to be demonstrated whether interventions later in life can modulate brain resilience. Several studies assessing the influence of different proxies of CR on the clinical manifestation of AD indicate that cognitive function is dynamic, rather than static [20]. This is congruent with the “use or lose it” paradigm [54]. According with Hultsch and co-workers [54] cognitive processes are considered modifiable also by exercise and experience, rather than innate factors. In this view, structural and functional brain changes are driven by the exposition to stimuli promoting learning and reinforcing the exiting neuronal connections [5]. In order to sustain the brain plasticity, and therefore to maintain the CR, cognitive rehabilitation in patients with early stage of AD can be considered a promising therapeutic target. This perspective assumes that a mentally stimulating lifestyle modifies the routes used by the brain to perform successfully a cognitive function. As a consequence, an intensive mental training might provide a greater resilience to cope with neuronal damage, likely providing alternative cognitive strategies or recruiting different brain networks [5].
Currently there is a rich literature on cognitive rehabilitation in AD patients [55], but there are no several studies that explore directly the effect of CR on cognitive outcome after rehabilitation. Olazarán and co-workers [56] only, investigating both AD and MCI patients, found better cognitive outcomes after cognitive stimulations in patients with low, compared with those with high educational level. This surprising result is likely due to the fact that in patients with high CR is needed accumulate more AD neuropathology to show same clinical symptoms of patients with low CR. The Authors [56] concluded that patients with high CR were less able to learn new generalizable strategies useful to improve cognitive performances. However, further studies assessing relationship between different CR levels and non-pharmacological treatments seem to be necessary and desirable in the immediate future.
Acknowledgment
This project has been supported by grants from the Italian Ministry of Health to Dr. Marco Bozzali (150/RF-2009-1491699; 047/RF-2010).
References
- Christensen DL, Baio J, Van Naarden Braun K., et al. (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill 65: 1 -23.
- Geschwind DH, Levitt P (2007) Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 17: 103-111. [Crossref]
- Lord C, Rutter M, A Le Couteur (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659-685. [Crossref]
- Gotham K, Risi S, Pickles A, Lord C (2007) The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 37: 613-627. [Crossref]
- Levy SE, Mandell DS, Schultz RT (2009) Autism. Lancet 374: 1627-1638. [Crossref]
- Wiggins LD, Baio J, Rice C (2006) Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr 27: S79-87. [Crossref]
- Retico A, Tosetti M, Muratori F, Calderoni S (2014) Neuroimaging-based methods for autism identification: a possible translational application? Funct Neurol 29: 231-239. [Crossref]
- Levman J, Takahashi E (2015) Multivariate analyses applied to fetal, neonatal and pediatric MRI of neurodevelopmental disorders. Neuroimage Clin 9: 532-544. [Crossref]
- Jin, Y., C. Y. Wee, F. Shi, K. H. Thung, D. Ni, P. T. Yap and D. Shen (2015). "Identification of infants at high-risk for autism spectrum disorder using multiparameter multiscale white matter connectivity networks." Hum Brain Mapp 36(12): 4880-4896.
- Plitt M, Barnes KA, Martin A (2015) Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. Neuroimage Clin 7: 359-366. [Crossref]
- Ecker C, Rocha-Rego V, Johnston P, Mourao-Miranda J, Marquand A, et al. (2010) Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage 49: 44-56. [Crossref]
- Sato JR, Hoexter MQ, Oliveira PP, Brammer MJ, Consortium MA, et al. (2013) Inter-regional cortical thickness correlations are associated with autistic symptoms: a machine-learning approach. J Psychiatr Res 47: 453-459. [Crossref]
- Gori I, Alessia G, Filippo M, Irene S, Piernicola O, et al. (2015) Gray Matter Alterations in Young Children with Autism Spectrum Disorders: Comparing Morphometry at the Voxel and Regional Level. J Neuroimaging 25: 866-874. [Crossref]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TL, et al. (2011) Functional connectivity magnetic resonance imaging classification of autism. Brain 134: 3742-3754. [Crossref]
- Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, et al. (2013) Multisite functional connectivity MRI classification of autism: ABIDE results. Front Hum Neurosci 7: 599. [Crossref]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, et al. (2013) Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 70: 869-879. [Crossref]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, et al. (2010) Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res 3: 350-358. [Crossref]
- Ingalhalikar M, Parker D, Bloy L, Roberts TP, Verma R (2011) Diffusion based abnormality markers of pathology: toward learned diagnostic prediction of ASD. Neuroimage 57: 918-927. [Crossref]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C (2011) EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med 9: 18. [Crossref]
- Duffy FH, Als H (2012) A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med 10: 64. [Crossref]
- Hampson DR, Blatt GJ (2015) Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci 9: 420. [Crossref]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL (1988) Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 318: 1349-1354. [Crossref]
- Yang X, Si T, Gong Q, Qiu L, Jia Z, et al. (2016) Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: A meta-analysis of voxel-based morphometry studies. Aust N Z J Psychiatry 50: 741-753. [Crossref]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, et al. (2002) Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59: 184-192. [Crossref]
- Herrington JD, Miller J, Pandey J, Schultz RT (2016) Anxiety and Social Deficits Have Distinct Relationships with Amygdala Function in Autism Spectrum Disorder. Soc Cogn Affect Neurosci 11: 907-914. [Crossref]
- Rausch A, Zhang W, Haak KV, Mennes M, Hermans EJ, et al. (2016) Altered functional connectivity of the amygdaloid input nuclei in adolescents and young adults with autism spectrum disorder: a resting state fMRI study. Mol Autism 7: 13. [Crossref]
- Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, et al. (2014) A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuropsychopharmacol Biol Psychiatry 48: 124-128. [Crossref]
- Sussman D, Leung RC1, Vogan VM1, Lee W1, Trelle S1, et al. (2015) The autism puzzle: Diffuse but not pervasive neuroanatomical abnormalities in children with ASD. Neuroimage Clin 8: 170-179. [Crossref]
- Foster NE, Doyle-Thomas KA, Tryfon A, Ouimet T, Anagnostou E, et al.(2015) Structural Gray Matter Differences During Childhood Development in Autism Spectrum Disorder: A Multimetric Approach. Pediatr Neurol 53: 350-359. [Crossref]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, et al. (2015) Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp 36: 4497-4511. [Crossref]
- Solso S, Xu R, Proudfoot J, Hagler DJ, Campbell K, et al. (2015) Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Biol Psychiatry 79: 676-684. [Crossref]
2021 Copyright OAT. All rights reserv
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, et al. (1999) The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport 10: 1647-1651. [Crossref]
- Sato W, Kubota Y, Kochiyama T, Uono S, Yoshimura S, et al. (2014) Increased putamen volume in adults with autism spectrum disorder. Front Hum Neurosci 8: 957. [Crossref]
- Jiao Y, Chen R, Ke X, Chu K, Lu Z, et al. (2010) Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage 50: 589-599. [Crossref]
- Bloy L, Ingalhalikar M, Eavani H, Roberts TP, Schultz RT et al. (2011) HARDI based pattern classifiers for the identification of white matter pathologies. Med Image Comput Comput Assist Interv 14: 234-241. [Crossref]
- Chen CP, Keown CL, Jahedi A, Nair A, Pflieger ME, et al. (2015) Diagnostic classification of intrinsic functional connectivity highlights somatosensory, default mode, and visual regions in autism. Neuroimage Clin 8: 238-245. [Crossref]
- Zurcher NR, Bhanot A, McDougle CJ, Hooker JM (2015) A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: current state and future research opportunities. Neurosci Biobehav Rev 52: 56-73. [Crossref]
- Carina Gillberg I, Bjure J, Uvebrant P, Vestergren E, Gillberg C (1993) SPECT (Single Photon Emission Computed Tomography) in 31 children and adolescents with autism and autistic-like conditions. Eur Child Adolesc Psychiatry 2: 50-59. [Crossref]
- Kaya M, Karasalihoglu S, Ustun F, Gultekin A, Cermik TF, et al. (2002) The relationship between 99mTc-HMPAO brain SPECT and the scores of real life rating scale in autistic children. Brain Dev 24: 77-81. [Crossref]
- Wilcox J, Tsuang MT, Ledger E, Algeo J, Schnurr T (2002) Brain perfusion in autism varies with age. Neuropsychobiology 46: 13-16. [Crossref]
- Ito H, Mori K, Hashimoto T, Miyazaki M, Hori A, et al. (2005) Findings of brain 99mTc-ECD SPECT in high-functioning autism--3-dimensional stereotactic ROI template analysis of brain SPECT. J Med Invest 52: 49-56. [Crossref]
- Sasaki M, Nakagawa E, Sugai K, Shimizu Y, Hattori A, et al. (2010) Brain perfusion SPECT and EEG findings in children with autism spectrum disorders and medically intractable epilepsy. Brain Dev 32: 776-782. [Crossref]
- Yang WH, Jing J, Xiu LJ, Cheng MH, Wang X, et al. (2011) Regional cerebral blood flow in children with autism spectrum disorders: a quantitative (9)(9)mTc-ECD brain SPECT study with statistical parametric mapping evaluation. Chin Med J (Engl) 124: 1362-1366.
- Amen DG, Hanks C, Prunella J (2008) Preliminary evidence differentiating ADHD using brain SPECT imaging in older patients. J Psychoactive Drugs 40: 139-146. [Crossref]
- Amen DG, Hanks C, Prunella J (2008) Predicting positive and negative treatment responses to stimulants with brain SPECT imaging. J Psychoactive Drugs 40: 131-138. [Crossref]
- Chang W, Henkin RE, Buddemeyer E (1984) The sources of overestimation in the quantification by SPECT of uptakes in a myocardial phantom: concise communication. J Nucl Med 25: 788-791. [Crossref]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273-289. [Crossref]
- Rothenberg TJ, Fisher FM, Tilanus CB (1964) A note on the estimation of the location parameters of the Cauchy distribution. J Amer Statistic Assoc 61: 852-855. [Crossref]
- Raji CA, Tarzwell R, Pavel D, Schneider H, Uszler M, et al. (2014) Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: a systematic review. PLoS One 9: e91088. [Crossref]
- Foster KR, Koprowski R, Skufca JD (2014) Machine learning, medical diagnosis, and biomedical engineering research - commentary. Biomed Eng Online 13: 94. [Crossref]
- Tibshirani R (1996) Regression Shrinkage and Selection via the lasso. Journal of the Royal Statistical Society 58: 267–88. [Crossref]
- Goldani AA, Downs SR, Widjaja F, Lawton B, Hendren RL (2014) Biomarkers in autism. Front Psychiatry 5: 100. [Crossref]
- Murdaugh DL, Shinkareva SV, Deshpande HR, Wang J, Pennick MR, et al. (2012) Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One 7: e50064. [Crossref]
- Wang H, Chen C, Fushing H(2012). Extracting multiscale pattern information of fMRI based functional brain connectivity with application on classification of autism spectrum disorders. PLoS One 7: e45502. [Crossref]
- Deshpande G, Libero LE, Sreenivasan KR, Deshpande HD, Kana RK (2013) Identification of neural connectivity signatures of autism using machine learning. Front Hum Neurosci 7: 670. [Crossref]
- Marcel AJ, Vladimir LC, Augusto B, Timothy AK, Tom MM (2014) Identifying autism from neural representations of social interactions: neurocognitive markers of autism. PLoS One 9: e113879. [Crossref]
- Zhou Y, Yu F, Duong T (2014) Multiparametric MRI characterization and prediction in autism spectrum disorder using graph theory and machine learning. PLoS One 9: e90405. [Crossref]
- Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A (2015) Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci U S A 112: E6699-6706. [Crossref]
- Gori I, Giuliano A, et al. (2015) Gray Matter Alterations in Young Children with Autism Spectrum Disorders: Comparing Morphometry at the Voxel and Regional Level. J Neuroimaging 25: 866-874. [Crossref]
- Stahl D, Pickles A, Elsabbagh M, Johnson MH; BASIS Team (2012) Novel machine learning methods for ERP analysis: a validation from research on infants at risk for autism. Dev Neuropsychol 37: 274-298. [Crossref]
- Jamal W, Das S, Oprescu IA, Maharatna K, Apicella F, et al. (2014) Classification of autism spectrum disorder using supervised learning of brain connectivity measures extracted from synchrostates. J Neural Eng 11: 046019. [Crossref]
- D'Mello AM, Stoodley CJ (2015) Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci 9: 408. [Crossref]
- Trevarthen C, Delafield-Butt JT (2013) Autism as a developmental disorder in intentional movement and affective engagement. Front Integr Neurosci 7: 49. [Crossref]
- Lombardo MV, Chakrabarti B, Bullmore ET, Consortium MA, Baron-Cohen S (2011) "Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism." Neuroimage 56: 1832-1838. [Crossref]
- Li W, Mai X, Liu C (2014) The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci 8: 74. [Crossref]
- Groen W, Teluij M, Buitelaar J, Tendolkar I (2010) Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry 49: 552-560. [Crossref]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, et al. (2000) The amygdala theory of autism. Neurosci Biobehav Rev 24: 355-364. [Crossref]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, et al. (2000) Abnormal regional cerebral blood flow in childhood autism. Brain 123: 1838-1844. [Crossref]
- Brennan JR, Wagley N, Kovelman L, Bowyer SM, Richard AE, et al. (2016). Magnetoencephalography shows atypical sensitivity to linguistic sound sequences in autism spectrum disorder. Neuroreport 27: 982-986. [Crossref]
- Leitner Y (2014) The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci 8: 268. [Crossref]