Abstract
N,N-DMT molecule is known in many cultures as endogenous hallucinogen and most research has been directed towards the psychological effects or structural definition while molecular interactions in conditions of the human body are poorly understood. We have complemented past structural research of N,N-DMT and investigated molecular interactions of N,N-DMT in the environment of 0.1 M hydrochloric acid which is characteristic to gastric fluid. Experimentally we have measured spectra of N,N-DMT with IR and Raman techniques and computed individual vibrational frequencies at DFT–B3LYP level of theory. Furthermore we put N,N-DMT in solution similar to gastric fluid environment and experimentally detected shift in vibrational frequency which is confirmed in our calculation. Our theoretical model shows that the cause of this shift is cation- π bond between N,N-DMT and HCl in the vicinity of the benzene ring.
Key words
Dimethyltryptamine, gastric acid, cation-π bond, hydrochloric acid, FTIR, Raman
Abbreviations
N,N-DMT: N,N-dimethyltryptamine; HCl: Hydrocloric acid, FTIR: Fourier transform infrared; IR: Infrared
Introduction
The N,N-dimethyltryptamine (N,N-DMT) is an endogenous compound, first synthesized in 1931. It was confirmed to have hallucinogenic properties in 1956. It was natural derived from the essential amino acid tryptophan and ultimately produced by the enzyme indolethylamine-N-methyltransferase (INMT) during normal metabolism [1]. Structurally speaking N,N-DMT consists of indole ring connected through propyl chain with dimethylamino group. Research proved his natural existence in the human blood and urine other mammals and wide range of plants [2-6]. Some authors have conducted research about therapeutic potential of this compound [7]. The latest research demonstrated that N,N-DMT has immunomodulatory potential that may contribute to significant anti-inflammatory effects and tissue regeneration [8]. In Brazil there are drinks containing N,N-DMT as ingredient and are legally used during the course of religious activities and for healing purposes [5]. During oral intake of those drinks, N,N-dimethyltryptamine interacts with hydrochloric acid inside the stomach. Interactions between N,N-dimethyltryptamine and hydrochloric acid within the gastric fluid are not widely researched on molecular level, so our motivation is to investigate these interactions of N,N-DMT and HCl, theoretically and experimentally.
Materials and methods
95%> pure N,N-dimethyltryptamine was purchased from AKos Consulting & Solutions GmbH company and used without further purification. The supplier of 37% Hydrocloric acid was Sigma-Aldrich. To simulate natural conditions in stomach we prepared experimental solution in a manner that we first diluted Hydrocloric acid with water to get natural concentration of HCl in gastric fluid [9,10] which is in our experiment 0,1M and then we put N,N-DMT in a solution. To observe interactions, solution was characterized by attenuated total reflectance Fourier transform infrared spectroscopy (ATR FTIR), Spectrum One FT-IR spectrometer, Perkin Elmer (model 2000), in the range from 4000 to 650 cm−1. The FT-IR spectrometer was connected to a PC with the installed IRDM (IR Data Manager) program to process the recorded spectra. FTIR spectra were normalized using baseline correction from software Essential FTIR. First we recorded spectrum of an aqueous solution sample of N,N-DMT and HCl, and then we put solution in a desiccator over silica gel for 24 hours and then we recorded dry sample. Pure N,N-DMT Raman spectrum from 4000–155 cm−1 was recorded on a Nicolet 6700 FT-IR spectrometer with NXR FT-Raman module coupled with an IBM AT computer. The 1064 nm YVO4 laser was used for excitation.
The quantum chemical calculations were performed with the Gaussian 09 package program at DFT–B3LYP level of theory [11]. The standard 6-311++G(d,p) basis set was used to carry out the calculations of molecular geometries, PE hypersurfaces, force fields, vibrational frequencies, as well as IR intensities and Raman scattering activities.
Results
Conformational analysis
We have found three stable conformations and we selected the lowest-energy conformation for further analysis. This conformation is shown in Figure 1. The potential energy has been scanned by changing the dihedral angle C12-C15-C16-N17 from 0° to 360° in one hundred steps, which is equivalent to rotation about C15-C16 bond. At each scanning step configuration was optimized.
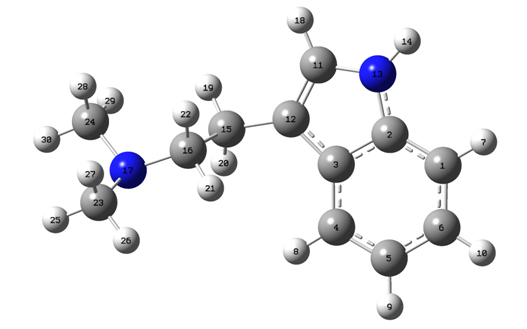
Figure 1. Most stable conformation of N,N-dimethyltyptamine
Vibrational analysis
N,N-Dimethyltyptamine: Objective of the vibrational analysis was to find vibrational modes connected with specific molecular structure of calculated molecule. Molecule was investigated using Raman spectroscopy (Figure 2) in the region of 50-3500 cm-1 and IR spectroscopy (Figure 3) in the region of 650-4000 cm-1.
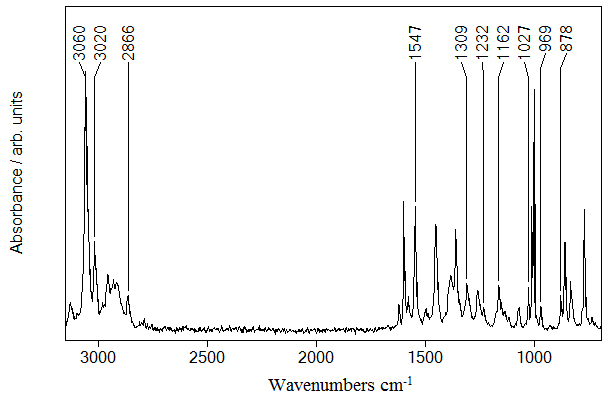
Figure 2. Raman spectrum of N,N-DMT in (3200–700) cm-1 region
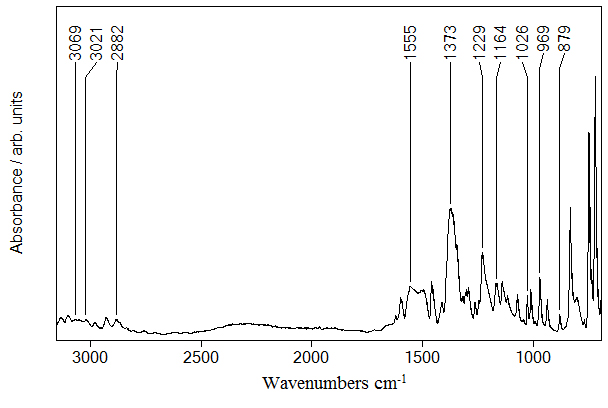
Figure 3. IR spectrum of N,N-DMT in (3200–650) cm-1 region
Calculated frequencies for conformation given in Figure 1 and experimental frequencies presented in Table 1 are grouped by vibrational modes of specific groups in molecule. Calculated C-H symmetrical stretching frequency is at 3188 cm-1 and C-H symmetrical antiphase stretching at 3166 cm-1. Other experimentally observed frequencies come from C-H asymmetrical ring stretching (C4H8, C5H9, C6H10,C1H7) at 3131 cm-1 in both IR and Raman spectra, while calculated peeks are at 3177 cm-1 and 3160 cm-1. We also observed C-H asymmetrical stretching vibrations of methyl group (C23H25, C23H26) at 3069 cm-1 with IR and 3060 cm-1, 3122 cm-1 with Raman. Calculated frequencies for this vibrational mode are 3106 cm-1 and 3095 cm-1. For C-H symmetrical stretching of methyl group calculated vibrations (C23H25, C23H26) are at 3064 cm-1 and 3052 cm-1 while experimental frequency is at 3020 cm-1 both in Raman and IR spectrum. Symmetrical CH2 stretching (C15H19H20) has been calculated at a frequency of 3026 cm-1 and found in spectra at 2980 cm-1 with both IR and Raman. C-H in phase stretching (C23H27, C24H28, C16H22) calculated frequencies for this mode is 2896 cm-1. There is also antiphase mode for the same C-H stretching with calculated frequency at 2890 cm-1 and experimentally found with both Raman and IR at 2866 cm-1. CC modes of aromatic stretching can be seen with both IR at 1599 cm-1 (C3C4, C5C6, C12C11), 1495 cm-1 (in ring C-C stretching), 1362 cm-1 (in ring C-C stretching), and with Raman at 1579 cm-1 (C11C12, C12C15, C2C1 coupled stretching), 1598 cm-1 (C3C4, C5C6, C12C11), 1498 cm-1 (C3C4, C4C5) and 1360 cm-1 (in ring C-C stretching). Calculated frequencies for these modes are at 1587 cm-1, 1657 cm-1, 1520 cm-1 and 1370 cm-1. N-C stretching coupled with C-C stretching is computed at 1295 cm-1 (N17C16),1275 cm-1 (N13C11), 1250 cm-1, 868 cm-1 (N17C16) and observed with IR 1262 cm-1, 1229 cm-1, 855 cm-1 and with Raman at 1259 cm-1, 1232 cm-1, 859 cm-1. Small amplitude N-H bending, coupled with C-H bending on the benzene ring is also present in calculated data at the frequency region of 1161 cm-1 and in experimental data at 1139 cm-1 in Raman spectra and at 1134 cm-1 in IR spectra. Calculated frequency for N-H bending (N13H14, C11H18) and stretching (N13C2) is 1240 cm-1. This mixed vibrational mode is experimentally observed in Raman spectrum at 1232 cm-1 and in IR spectrum at 1229 cm-1. Other vibrational type we computed is C-H bending coupled with C-C stretching at 1035 cm-1, 1029 cm-1 (C16H21, C15H19), 1022 cm-1. These peaks were experimentally observed with both IR and with Raman at 1027 cm-1. Last group of assigned frequencies are out of ring plane C-H bendings computed at 746 cm-1, 641 cm-1, 584 cm-1, 429 cm-1 and found with IR at 735 cm-1 and with Raman at 734 cm-1, 617 cm-1, 561 cm-1, 409 cm-1.
|
Teoretical frequency
cm-1
|
Experimental
frequency cm-1
|
Vibrational mode
|
|
IR
|
Raman
|
|
3188
|
-
|
-
|
CH symmetrical stretching
|
|
3166
|
-
|
-
|
CH symmetrical stretching antiphase
|
|
3177
|
-
|
-
|
CH asymmetrical ring stretching
|
|
3160
|
3132
|
3131
|
CH asymmetrical ring stretching
|
|
3238
|
-
|
-
|
CH stretching
|
|
3106
|
-
|
3122
|
CH asymmetrical stretching methyl group
|
|
3095
|
3069
|
3060
|
CH asymmetrical stretching methyl group
|
|
3064
|
-
|
-
|
CH symmetrical stretching methyl group
|
|
3052
|
3020
|
3020
|
CH symmetrical stretching methyl group
|
|
3026
|
2980
|
2980
|
CH2 symmetrical stretching methyl group
|
|
2896
|
-
|
-
|
CH stretching coupled with N
|
|
2890
|
2867
|
2866
|
CH stretching coupled with N in antiphase
|
|
1657
|
1599
|
1598
|
C-C stretching
|
|
1587
|
-
|
1579
|
C-C stretching
|
|
1520
|
1495
|
1498
|
C-C stretching
|
|
1370
|
1362
|
1360
|
C-C stretching
|
|
1295
|
1262
|
1259
|
N-C, C-C stretching
|
|
1275
|
1229
|
1232
|
N-C, C-C stretching
|
|
1250
|
-
|
-
|
N-C, C-C stretching
|
|
868
|
855
|
859
|
N-C, C-C stretching
|
|
1240
|
1229
|
1232
|
NH bending
|
|
1177
|
-
|
-
|
NH bending CH
|
|
1161
|
1139
|
1134
|
NH bending CH
|
|
1035
|
-
|
-
|
C-H bending coupled with C-C-stretching
|
|
1029
|
1027
|
1027
|
C-H bending coupled with C-C-stretching
|
|
1022
|
-
|
-
|
C-H bending coupled with C-C-stretching
|
|
746
|
735
|
734
|
C-H bending out of plane
|
|
641
|
-
|
617
|
C-H bending out of plane
|
|
584
|
-
|
561
|
C-H bending out of plane
|
|
429
|
-
|
409
|
C-H bending out of plane
|
Table 1. Experimental and computed values of vibrational frequencies for N,N-dimethyltryptamine
Discussion
Hydrocloric acid molecules with intermolecular hydrogen bonding have stretching band around 2930 cm-1. Various clusters of HCl can be formed by intermolecular hydrogen bond which shifts the only HCl stretching mode to lower frequencies. The shift is about 100 cm-1 and is observed in (Figure 4) as 2854 cm-1 band. The frequency band around 2350 cm-1 very probably originates from HCl clusters encapsulating one molecule of water. The calculations shows that such HCl clusters exist and the calculated frequency is around 2350 cm-1. On Figure 4 we can see frequencies around 1700-1900 cm-1 belonging to combination and overtone bands that are typical for benzenoid compounds [12]. We argue that HCl molecule interacts with N,N-DMT through cation-π type of interaction. It is well known that electrons in the benzene ring are delocalized and can form a new type of bond with electron deficient atom, which in our case is hydrogen atom of HCl molecule. This chemical bond shifts the frequency to the lower value. Therefore we have noticed that the measured frequency of this vibration in the spectra of pure Dimethyltryptamine was 1599 cm-1 and in the spectra of Dimethyltryptamine in hydrochloric acid solution is shifted to 1546 cm-1 in Figure 5. The experimental data shows that the frequency shift of stretching in benzene ring is about 53 cm-1. Calculation of this frequency shift with B3LYP functional indicates, but generally underestimates this shift. The idea is to put molecules of hydrochloric acid in the vicinity of benzene ring and optimize the whole structure composed of DMT and HCl molecules. As an example we are presenting structure shown in Figure 7. The calculated shift is 7 cm-1 and is obtained with four HCl molecules agglomerated in the vicinity of benzene ring. We argue that calculated shift with more than four HCl molecules could be even greater, having in mind that calculated shift is slighter when we used smaller number of HCl molecules. The same rule applies to low frequency C-H out of plane bending modes. However, the calculated frequency shift is toward higher frequencies in contrast to before mentioned C-C ring stretching frequency shift. These two opposite tendencies could be understood if we take into account that the hydrochloric interacts with delocalized electrons in the ring and thus weakening C-C bonds in contrast to hydrogen atoms bonded to ring which through additional interaction with chlorine atom stiffen their bonds with carbon atoms. We can corroborate this interpretation with experimental data in Figure 6 where we clearly see one very broad band around 750 cm-1. We argue that this broad band comes from both sides of the peak; frequency modes lower than 750 cm-1 are shifted toward higher, and higher frequency modes are shifted to lower frequencies.
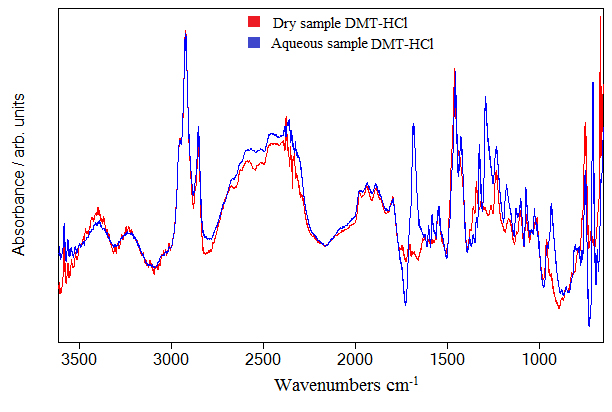
Figure 4. IR spectrum of DMT-HCl in (4000–600) cm-1 region
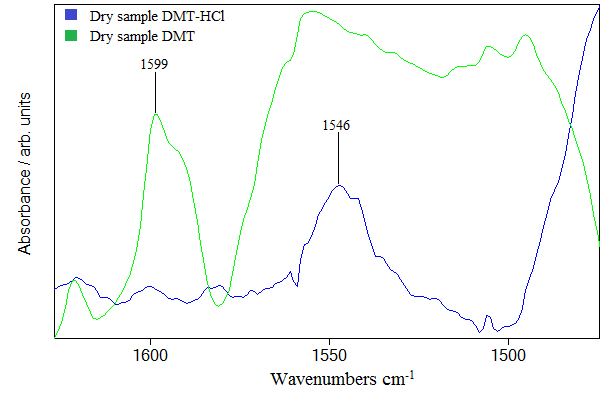
Figure 5. Shift in the IR spectrum in (1650–1460) cm-1 region
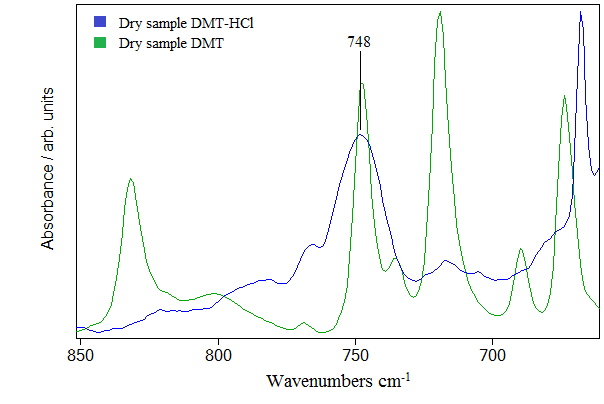
Figure 6. Shift in the IR spectrum in (650–850) cm-1 region
We should also take into account that we are working here with unscaled frequencies. The scaling factors would bring closer calculated and observed frequencies. The interaction between DMT and HCl can be very complex. It includes cation-π interaction between HCl and benzene ring and hydrogen bonding between HCl molecules. The calculated configuration shown in Figure 7 is just one possible simple example of DMT-HCl interaction, as we expect much more complex configurations due to higher concentrations of HCl and DMT molecules.
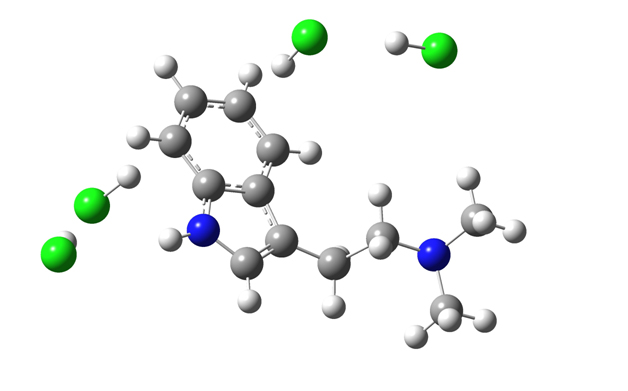
Figure 7. An example of DMT-HCl interaction in the area of benzene ring. Chlorine atoms are painted in green.
It is obvious that the calculations confirm experimentally observed shift which is a strong indication that cation-π interaction exists. When we had left N,N-DMT inside HCl solution of 0.1 M over a period of several hours up to a few days we didn’t detect the presence of N,N-DMT molecule. The significance of these facts in biology and medicine is that the HCl is an inhibitor of N,N-DMT activity and that it is the main agents for the decomposition of that molecule in the gastric fluid. Therefore, such an influence of HCl significantly reduces efficiency of N,N-DMT at oral intake.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. These authors contributed equally.
Acknowledgment
We thank Maja Lovković for the valuable contribution to data of Raman spectroscopy. We thank dr.sc. Zvonimir Katanačić and dr. sc. Ljerka Kratofil Krehula from Faculty of Chemical Engineering and Technology, University of Zagreb, for the valuable contribution to data of IR spectroscopy. We thank prof. dr. sc. Krešimir Košutić from Faculty of Chemical Engineering and Technology, University of Zagreb, for logistic support in the study.
2021 Copyright OAT. All rights reserv
References
- Axelrod J (1961) Enzymatic formation of psychotomimetic metabolites from normally occurring compounds. Science 134: 343.[Crossref]
- Forsström T1, Tuominen J, Karkkäinen J (2001) Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scand J Clin Lab Invest 61: 547-56. [Crossref]
- Kärkkäinen J, Forsström T, Tornaeus J, Wähälä K, Kiuru P, et al. (2005) Potentially hallucinogenic 5-hydroxytryptamine receptor ligands bufotenine and dimethyltryptamine in blood and tissues. Scand J Clin Lab Invest 65: 189-99.
- Barker SA, Borjigin J, Lomnick I, Strassman R (2013) LC/MS/MS analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate”. Biomedical Chromatography 27: 1690–1700.
- Gaujac A, Martinez ST, Gomes AA, De Andrade SJ, Da Cunha Pinto A et al. (2013) Application of analytical methods for the structural characterization and purity assessment of N,N-dimethyltryptamine, a potent psychedelic agent isolated from Mimosa tenuiflora inner barks. Microchemical Journal 109: 78–83.
- Pachter IJ, Zacharias DE, Ribeiro O (1959) Indole Alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostiles. J Org Chem 24: 1285–1287.
- Strassman R (2001) DMT: The Spirit Molecule: A Doctor's Revolutionary Research into the Biology of near-Death and Mystical Experiences, Rochester, Vt: Park Street.
- Szabo A, Kovacs A, Frecska E, Rajnavolgyi E (2014) Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS One 29: 9(8). [Crossref]
- Di Mario F, Goni E (2014) Gastric acid secretion: changes during a century. Best Pract Res Clin Gastroenterol 28: 953-965. [Crossref]
- Guyton AC, John E (2006) HallTextbook of Medical Physiology, Elsevier Saunders, Philadelphia 797.
- Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB (2009)
- Silverstein RM, Webster FX, Kiemle D, Bryce DL (2005) Spectrometric Identification of Organic Compounds, Wiley, New York 72-119.







