Desmoid tumours (DT) are benign tumours that arise from proliferation of well differentiated fibroblasts which have potential to be locally aggressive and recur post excision. DTs can present both intra-abdominal or extra-abdominal locations with worse prognosis intra-abdominally. We present the case of a 59-year-old gentleman with 4-week history of right abdominal mass with mild discomfort but no gastrointestinal symptoms. He underwent investigations following which underwent excision of 3 masses. Association of intra-abdominal DT with FAP is well documented.Intra-abdominal DTs are more prevalent in females with history of surgery. Our case is unique as our patient does not confer to the above categories
Desmoid Tumours (DT), intra-abdominal, asymptomatic patient
Symptoms from DTs may arise as the results of mass effect or compression on abdominal organs. This effect may be manifested by intestinal obstruction, urinary obstruction or rarely arterial or venous compression FAP (Familial Adenomatous Polyposis), results from mutation of the APC gene and accounts for 1% cases of colorectal cancer and account for 0.03% of neoplasms [1]. DT account for less than 3% of all soft tissue tumours. They occur most often between 15-60 years of age with being rare in the young and in the elderly [2,3]. The incidence of DT is approximately 2-4 per million populations per year in United States. They have slightly greater preponderance in women than in men. DT occurs in 10-20% of patients with FAP [4]. A patient with FAP has an 852-fold increased risk of developing a DT [4].
Mr P, a 59-year old gentleman previously fit and well presented with a 4-week history of right abdominal mass associated with mild discomfort in June 2015 to the surgical outpatient clinic. There was no reporting of any associated gastrointestinal symptoms. The mass was noted over the paramedian incisional scar through which he had undergone laparoscopic to open appendicectomy for a complicated appendicitis in 2013. There was no other related past medical history of relevance. Family history was limited from the maternal aspect as he had been adopted.
Investigation/Plan |
Results/Outcome |
CT Abdomen+Pelvis |
6.5 cm soft tissue mass in the lower abdomen below level of umbilicus (See Image.1) |
USS Abdomen |
67mm x 63mm x 65mm, with peripheral vascularity seen (See Image.2) |
Ultrasound guided biopsy of mass- transcutaneous |
Fibrous connective tissue,formed of fascicles and bundles of spindled cells with spindle or comma shaped nuclei and wavy cytoplasm. There was no evidence of malignancy |
Multidiscplinary Team discussion |
Decision for excision of lesion |
Laporotomy and excision of small bowel mesentery masses |
Findings intra-operatively:-10 cm mass was noted in the mesentery of the proximal ileum at juxta position to the serosa of the small bowel. Furthermore, there were a smaller (5 cm) similar mass in the distal jejunum and a 2 cm similar mass about 20 cm proximal to the largest lesion, all 3 lesions embedded in the mesentery and located at serosal juxta positions. The smallest mesenteric mass was enucleated and sent for frozen section prior to formal resection
|
Histology |
Frozen section- consistent with mesenteric fibromatosis (DT),
Formal Histology-The tumours have a similar appearance of very bland fibrosis with bundles of eosinophilic cells and small blood vessels with focal keloid fibres.. No necrosis, abnormal mitoses or nuclear pleomorphism is seen throughout these masses.All lymph nodes were noted to be clear. Initial immunohistochemistry shows negative staining with CD117 and DOG1 as well as CD34. Cytokeratin, Desmin, S100 and SMA are all negative in the larger tumour while Vimentin is positive. There is some focal positivity for SMA in the smaller specimen but CD117 and DOG1 are universally negative. Beta catenin staining, carried out in Glasgow shows strong nuclear positivity.Both the light microscopic appearances and the immunohistochemistry are in keeping with mesenteric fibromatosis (desmoid tumour). |
National Sarcoma MDT |
Post-operative CT scan, colonoscope and UGI endoscope |
CT Thorax, Abdomen and Pelvis- Follow up scan |
Low volume mesenteric lymph nodes but no DTs seen. (See Image. 3) |
He was subsequently assessed by the oncology team specialised in management of soft tissue tumours in the West of Scotland. The outcomes are in the section titled follow-up (Figures 1-3).
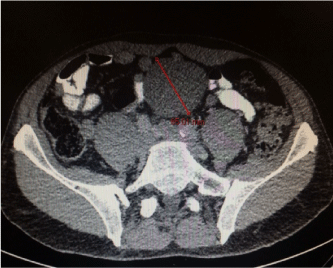
Figure 1. CT scan of the Abdomen and Pelvis. There is well demarcated lesion measuring 6.5 cm in maximal diameter.
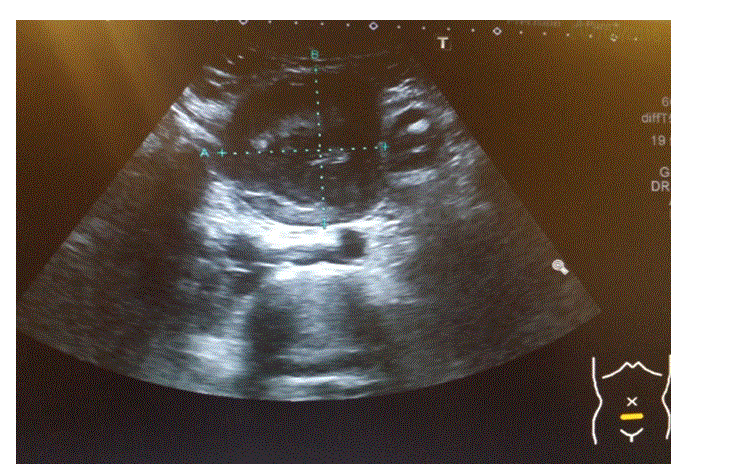
Figure 2. US scan of the Abdomen.
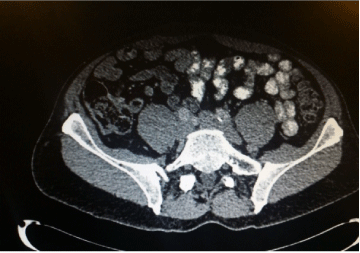
Figure 3. CT scan of Abdomen and Pelvis. Post. Operatively. Note no resdiual masses visible.Only residual mesenteric lymph nodes visible on this CT scan (Bright areas)
The exact aetiology of DT is unclear. The vast majority of DT occurs extra-abdominally on the peripheral areas of the body for e.g. shoulders, gluteal regions. The vast majority (85%) of intra-abdominal DT occur in the mesentery [5]. Sturt et al. [6] studied for the evidence for genetic predisposition to desmoids tumours formation in FAP from patients from the polyposis registry at Middlesex Hospital in the UK. The results indicated that the influence of unknown genetic factors independent of germline APC mutations were associated with formation of desmoids tumours. Desmoid tumours of sporadic nature are associated with oestrogen [7] whilst the role of oestrogen in the aetiology of FAP related associated desmoids tumours are less well documented. Differential diagnoses for intra-abdominal DT would be gastrointestinal stromal tumour (GIST), solitary fibrous tumours (SFT), inflammatory myofibroblastic tumour. Histologically the differentiation between GIST and DT is noted to be straightforward, in GIST it is c-KIT positive whilst in DT this is negative.
Guidelines as proposed by the National Comprehensive Cancer Network (NCCN), suggested patients with small tumours to have regular observation of the tumour especially if any surgery would lead to significant morbidity [7]. Fiore et al. [8] retrospectively studied cases of intra-abdominal DT and found that regular observation benefited half of the patients being studied.
There are various guidelines for the management of soft tissue sarcoma including that of DTs. The two major recommendations that were looked at were from the European Society for Medical Oncology (ESMO) (European) and the National Comprehensive Cancer Network (NCCN) (Normal American). Both organisations suggest a Multidisciplinary team (MDT) approach with expertise in sarcoma.
According to the ESMO Clinical Practice Guidelines [9] for soft tissue sarcomas, watchful waiting policy is applicable after shared decision has taken place to exclude of its presence in potentially life-threatening extra-abdominal locations and intra-abdominal locations. It goes on to state that treatment is reserved for progressing cases. The preferred imaging is with Magnetic Resonance Imaging (MRI) although the tumour signal is not necessarily indicative of disease evolution.
Primary surgery with complete resection with negative margins is the treatment of choice for DTs. However positive margins post-surgery indicates a high risk of recurrence [10]. The role of radiation is well documented in the treatment of DTs including in patients not suitable for operative management. Use of neoadjuvant radiotherapy is not well established, largely due to unavailability of large trials. With regards to post-surgical radiation therapy the benefits are limited [11].
Pharmacologic agents result in variable rates of response approximately 40-50% [12] depending on the duration of treatment given. This includes use of an NSAID, anti-oestrogen therapy or a combination of both. The role of chemotherapy in treatment of DTs is well documented. Agents commonly used include doxorubicin, dacarbazine and carboplatin.
Clinical State
|
NCCN Guidelines [13]
|
ESMO Guidelines [9]
|
Asymptomatic patient, not in a lifethreatening location*
|
Careful watch and wait
|
Careful watch and wait
|
Progressive cases and patient suitable for surgery
|
Resection and/or RTX and/or systemic therapy
|
Wide excision following standard tx, high grade deep lesions> 5cm, RTX if tumour is within the resected compartment
|
Symptomatic patient
|
Surgery and/or radiation and /or systemic therapy
|
For progressing cases, surgery is indicated (without any adjuvant therapy), radiation therapy and observation
|
Systemic therapy
|
Sorafenib and NSAIDs, hormonal and biologic agents (tamoxifen, low-dose INF) chemotherapy agents such as Vinblastine and Methotrexate
|
Hormonal therapies e.g. tamoxifen, GnRH analogues, NSAIDs, low-dose chemotherapy (e.g. methotrexate +vinblastine or methotrexate +vinorelbine, full dose chemotherapy
|
* potentially life threatening extra-abdominal locations (e.g. Head and neck region) and intra-abdominal desmoids (mesenteric fibromatosis).
Follow-up
ESMO Guidelines [9]
2021 Copyright OAT. All rights reserv
- Cases with surgical resection- intermediate/high grade cases: -
- Every 3-4 months for the first 2-3 years
- Twice a year- up to 5 years
- Thereafter once a year
- Low grade cases: -
Every 4-6 months with CXR/CT scan in the first 3-5 years
Thereafter once a year
NCCN Guidelines [13]
- Careful history, examination and imaging every 3-6 months for 2 years
- Thereafter once a year
It can be seen both the ESMO and the NCCN guidelines with regards to DTs highlight similar modalities of treatment. The Scottish Sarcoma Network has proposed clinical management guideline for fibromatosis (DT) for adults older than 16 yrs (Figures 4 and 5).
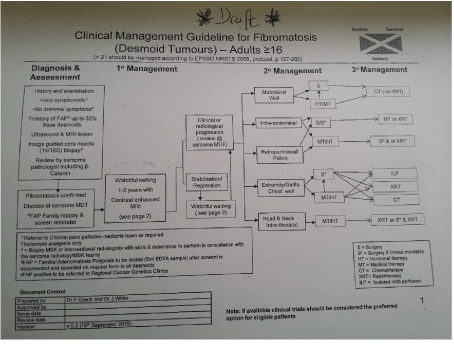
Figure 4. Proposed Flowchart from SCCN on management of desmoid tumours.

Figure 5. Proposed Flowchart from SCCN on management of desmoid tumours.
They proposed the clinical management guidelines [14] for fibromatosis (DT) from the data from the European Consensus Data. According to the guidelines Mr P should be under surveillance initially for periods of 1-2 years with imaging with contrast enhanced MRI. In discussion with the lead author for the proposed guideline, the proposed guidleines incorporate the findings from the recomendations from the European Journal of Cancer.
In our patient, 2 of the 3 DT were less than 10 cm in maximal measurement, however due to its location (intra-abdominal) and possibility of early bowel obstruction the decision to operate was undertaken. According to the ESMO guidelines, the location of the DT would be classified as life-threatening when there is a risk of bowel obstruction due to significant growth of the tumour.
As seen from the literature DTs contribute to a small proportion of all neoplasms. There is an association of intra-abdominal DT with FAP. The other group where intra-abdominal DT are more prevalent are in females with history of previous surgery. Our case is unique as our patient does not fall into any of the above categories. The basic management of intra-abdominal DT is primary resection with any adjuvant therapy as deemed appropriate by the MDT specialising in soft tissue tumours.
The authors for this article declare that they have no conflicts of interest. The authors are solely responsible for the information and the write up of this article.
The authors for this article have received no grants or funds for this article from any institutions or educational bodies.
The authors would like to thank the patient for allowing his case to be shared for educational purposes.
- Akshoomoff N (2006) Use of the Mullen Scales of Early Learning for the assessment of young children with Autism Spectrum Disorders. Child Neuropsychol 12: 269-277. [Crossref]
- Sparrow SS, Cicchetti D, Balla D (2005) Vineland adaptive behavior scales. (2ndedn) (Vineland-II). Circle Pines, MN: AGC Publishing.
- Rinz CJ, lennon VA, James F, Thoreson JB, Tsai k, et al. (2015) A CHRNE frameshift mutation causes congenital myasthenic syndrome in young Jack Russell Terriers. Neuromuscul Disord 25: 921-927. [Crossref]
- Kinali M, Beeson D, Pitt MC, Jungbluth H, Simonds AK, et al. (2008) Congenital myasthenic syndromes in childhood: diagnostic and management challenges. J Neuroimmunol 201-202: 6-12. [Crossref]
- Engel AG, Shen XM, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14: 461. [Crossref]
- Engel AG1 (2008) Congenital myasthenic syndromes. Handb Clin Neurol 91: 285-331. [Crossref]
- Webster R, Liu WW, Chaouch A, Lochmüller H, Beeson D (2014) Fast-channel congenital myasthenic syndrome with a novel acetylcholine receptor mutation at the a-e subunit interface. Neuromuscul Disord 24: 143-147. [Crossref]
- Azuma Y, Nakata T, Tanaka M, Shen XM, Ito M, et al. (2015) Congenital myasthenic syndrome in Japan: ethnically unique mutations in muscle nicotinic acetylcholine receptor subunits. Neuromuscul Disord 25: 60-69. [Crossref]
- Engel AG, Shen XM, Selcen D, Sine S (2012) New horizons for congenital myasthenic syndromes. Ann N Y Acad Sci 1275: 54-62. [Crossref]
- Schara U, Christen HJ, Durmus H, Hietala M, Krabetz K, et al. (2010) Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Paediatr Neurol 14: 326-333. [Crossref]
- Levin ED1 (2002) Nicotinic receptor subtypes and cognitive function. J Neurobiol 53: 633-640. [Crossref]
- Selcen D, Shen XM, Brengman J, Li Y, Stans AA, et al. (2014) DPAGT1 myasthenia and myopathy: genetic, phenotypic, and expression studies. Neurology 82: 1822-30. [Crossref]
- Shen XM, Selcen D, Brengman J, Engel AG (2014) Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology 83: 2247-2255. [Crossref]
- Robinson EB, Neale BM, Hyman SE (2015) Genetic research in autism spectrum disorders. Curr Opin Pediatr 27: 685-691. [Crossref]
- Schanen NC (2006) Epigenetics of autism spectrum disorders. Hum Mol Genet 15 Spec No 2: R138-150. [Crossref]
- Sahin M, Sur M (2015) Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science 350. [Crossref]
- Gonzalez-Gronow M, Cuchacovich M, Francos R, Cuchacovich S, Blanco A, et al. (2015) Catalytic autoantibodies against myelin basic protein (MBP) isolated from serum of autistic children impair in vitro models of synaptic plasticity in rat hippocampus. J Neuroimmunol 287:1-8. [Crossref]
- Lord C, Bishop S, Anderson D (2015) Developmental trajectories as autism phenotypes. Am J Med Genet C Semin Med Genet 169: 198-208. [Crossref]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, et al. (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70: 863-885. [Crossref]
- De Rubeis S1, Buxbaum JD2 (2015) Genetics and genomics of autism spectrum disorder: embracing complexity. Hum Mol Genet 24: R24-31. [Crossref]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, et al. (2011) Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70: 886-897. [Crossref]
- Krumm N, Turner TN, Baker C, Vives L, Mohajeri K, et al. (2015) Excess of rare, inherited truncating mutations in autism. Nat Genet 47: 582-588. [Crossref]
- Zhan QT, Pan PP, Xu XR, Lou HY, Lou YY, et al. (2013) An overview of studies on psychological well-being in children born following assisted reproductive technologies. J Zhejiang Univ Sci B 14: 947-960. [Crossref]
- Bay B, Mortensen EL, Hvidtjørn D, Kesmodel US (2013). Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. BMJ 347: f3978.
- Sandin S1, Nygren KG, Iliadou A, Hultman CM, Reichenberg A (2013) Autism and mental retardation among offspring born after in vitro fertilization. JAMA 310: 75-84. [Crossref]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61-73. [Crossref]
- de Graaf-Peters VB1, Hadders-Algra M (2006) Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 82: 257-266. [Crossref]
- Felch AC, Granger RH (2008) The hypergeometric connectivity hypothesis: divergent performance of brain circuits with different synaptic connectivity distributions. Brain Res 1202: 3-13. [Crossref]
- Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C (2011) Factors affecting obstetric outcome of singletons born after IVF. Hum Reprod 26: 2878-2886. [Crossref]
- Magli MC, Gianaroli L, Ferraretti AP, Gordts S, Fredericks V, et al. (2009) Paternal contribution to aneuploidy in preimplantation embryos. Reprod Biomed Online 18: 536-542. [Crossref]





