Macroautophagy (hereafter referred to as autophagy) is a highly conserved cellular process that delivers proteins and organelles to the lysosome and controls the degradation of these substrates to facilitate homeostasis. In addition, it is an important process to adapt the availability of nutrients. Amino acids activate mammalian target of rapamaycin complex 1 (mTORC1), which is the key regulator of the autophagy signaling pathway. The depletion of amino acids negatively regulates mTORC1 and induces autophagy. Recent studies have shown that amino acids recruit mTORC1 to the lysosome by affecting vacuolar-type H+-ATPase (v-ATPase) and Rag guanosine triphosphatase A/B (RagA/B), thereby leading to the activation of mTORC1 on the lysosome and the inhibition of autophagy. Here, we review recent advances in the understanding of autophagy signaling by amino acids and their metabolites.
autophagy, amino acid, mTOR, v-ATPase, RagA, sestrin
Autophagy degrades cellular cytosolic components by delivering them to the lysosome and is a highly conserved catabolic process in organisms ranging from yeasts to mammals [1,2]. Autophagy plays an important role in basic biological functions, such as intracellular clearance of defective proteins and organelles, differentiation, and development [3-5]. Dysfunctions in autophagy are associated with severe diseases, such as heart disease, neurodegenerative disorders, and cancers [4,6,7]. There are three distinct types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy (hereafter referred to as autophagy) comprises bulk degradation and a multi-step process by which the portions of the cytoplasm and/or organelles are sequestered in a double-layered membrane structure called the autophagosome. This autophagosome then fuses with a lysosome for degradation. The autophagosome–lysosome structure is called the autolysosome [5]. For many years, it has been known that autophagy is activated by starvation, including amino acid depletion and it controls the concentration of free amino acids [8]. Although the detailed mechanism related to the control of autophagy by amino acids has not been completely clarified, it has been shown that the mammalian (or mechanistic) target of rapamycin complex 1 (mTORC1) is the key regulator of autophagy by amino acids [9-12]. In this review, we focus on the regulation of autophagy by amino acids and their metabolites as well as recent advances in studies of the regulation of mTORC1 by amino acids.
The processes involved in autophagy include the stages of initiation, during which an isolation membrane (phagophore) is formed, elongation and closure, during which a complete autophagosome is formed, and maturation, during which an autolysosome is formed by the fusion of an autophagosome and a lysosome [5]. It has been reported that amino acid depletion induces autophagy. However, recent studies indicated that various stimulations, including stress, organelle damage, hypoxia, and reactive oxygen species induce autophagy [13]. In autophagy, the key regulator is mTORC1, which controls the initiation stage of autophagy [14]. mTORC1 comprises mTOR, which is a serine/threonine kinase and the main active component, mammalian lethal with SEC13 protein 8 (mLST8, which is also called GbL) [15], DEP domain-containing mTOR-interacting protein (DEPTOR) [16], proline-rich Akt substrate of 40 kDa (PRAS40) [17], regulatory-associated protein of mTOR (Raptor) [18,19], and Tti1-Tel2 [20]. mTORC1 negatively regulates autophagy by repressing the Unc-51-like kinase (ULK)1/2 complex, which is an initial regulator of autophagy. The ULK1/2 complex comprises ULK1/2, ATG13 [21], and focal adhesion kinase family interacting protein of 200 kD (FIP200) [22]. When cells are in nutrient-rich conditions, mTOR gets activated and inhibits the kinase activity of ULK1/2. On the other hand, when cells are in amino acid-depleted conditions, mTOR is inactivated and ULK1/2 is activated. Activated ULK1/2 autophosphorylates and then phosphorylates ATG13 and FIP200, and then produces the phagophore to initiate autophagy. In yeast, ATG8 plays an important role in the elongation and closure stage, during which an autophagosome is formed. The ATG12–ATG5–ATG16 complex conjugates phosphatidylethanolamine (PE) with ATG8 to produce ATG8-PE. ATG8-PE mediates membrane fusion and elongates the phagophore to complete autophagosome formation. In addition, ATG8 brings substrates, such as aggregated proteins or damaged organelles, into an autophagosome [1]. Mammalian orthologs of yeast ATG8 have been identified, which are divided into the microtubule-associated protein 1 light chain 3 (LC3) subfamily and the g-aminobutyric-acid-type-A receptor-associated protein (GABARAP) subfamily. The LC3 subfamily contains LC3 alpha (LC3A), LC3 beta (LC3B), and LC3C. The GABARAP subfamily contains GABARAP, GABARAP-like 1 (GABARAPL1), and GABARAP-like 2 (GABARAPL2) [23-27]. LC3B has been studied most extensively in these subfamilies. Similar to yeast ATG8, LC3B is conjugated to PE via the cooperation of the ATG12–ATG5–ATG16 complex to form an autophagosome [26]. The membrane sources of the autophagosome include the endoplasmic reticulum [28,29], mitochondrial membrane [30], plasma membrane [31], Golgi [32], and recycling endosomes [33]. After autophagosome formation is complete, the autophagosome fuses with a lysosome to form an autolysosome. The fusion between an autophagosome and a lysosome is mediated by Rab7 [34,35], homotypic fusion and protein sorting (HOPS) complex [36], UV radiation resistance-associated gene protein (UVRAG) [37], and soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) [38-40]. A recent study indicated that the phosphorylation of LC3B by serine/threonine kinase (STK) 3 and STK4 is also necessary for the fusion of an autophagosome and a lysosome [41]. On the other hand, mTORC1 phosphorylates UVRAG to inhibit the fusion of an autophagosome with a lysosome [42], indicating that mTORC1 negatively regulates the autophagy process not only at the initiation stage but also during the maturation stage. Finally, the enzymes in the lysosomes or autolysosomes degrade the substrates brought by the autophagosomes.
Amino acids are transported into the cell from outside by solute carrier (SLC) superfamily proteins, which are membrane-spanning amino acid transporter proteins [43]. It has been reported that amino acids (particularly leucine, glutamine, or arginine) activate mTORC1, thereby blocking the autophagy pathway [9-12]. However, it is still unclear whether mTORC1 signaling is affected by one specific amino acid or by a combination of amino acids. Some amino acid transporters are antiporters, which require an extra amino acid for transport. For example, glutamine is imported into the cell by SLC1A5, and the imported glutamine is then exported by the bidirectional amino acid transporter SLC7A5, which transports the extracellular essential amino acids into cells [11,44]. This indicates that extracellular amino acid signaling is not the same as intracellular amino acid signaling. Therefore, it is difficult to separate the signals of amino acids received from outside the cells. In addition, amino acid metabolites regulate mTORC1 signaling. Nitric oxide (NO) and citrulline are produced from arginine by NO synthase. NO inhibits autophagy via the S-nitrosylation of JNK1 and IKKb. JNK1 phosphorylates Bcl-2, which binds and inhibits Beclin 1. Phosphorylated Bcl-2 releases Beclin 1, which then initiates autophagosome formation. In addition, IKKb phosphorylates AMPK, which then phosphorylates tuberous sclerosis 2 (TSC2). Phosphorylated TSC2 is a negative regulator of mTORC1, which induces autophagy [45]. Citrulline stimulates the phosphorylation of 4EBP1 and rpS6, which indicates mTORC1 activities; thus, citrulline is considered to be a candidate regulator of autophagy [46]. The metabolization of glutamine, which is called glutaminolysis, is processed by glutaminase (GLS) and glutamate dehydrogenase (GDH). GLS catalyzes glutamine to generate glutamate and ammonia. GDH catalyzes glutamate to generate a-ketoglutarate (aKG) and ammonia. It has been reported that ammonia induces autophagy, but the induction of autophagy by ammonia is independent of the mTORC1 and ULK1/2 signaling pathway [47-49]. By contrast, aKG activates mTORC1 signaling and blocks autophagy [50,51]. The metabolization of arginine, histidine, or proline also generates KG. Therefore, the balance between aKG and ammonia may be important in the regulation of autophagy. Specific leucine metabolites do not induce mTORC1 signaling [52]; however, leucine activates GDH via allosteric regulation to increase glutaminolysis, which regulates mTORC1 [51]. Thus, amino acids and their metabolites may affect mTORC1 and autophagy via complex signaling pathways.
mTORC1 is recruited to the lysosome and is activated by GTP-loaded Ras homolog enriched in brain (Rheb) on the lysosome. Several protein complexes play important roles in activating mTORC1 on the lysosome by amino acids (Figure 1). The biological substances that affect mTORC1 and autophagy are summarized in Table 1.
Table 1. Biological substances with effects on mTORC1 and autophagy
Biological substances |
mTORC1 |
Autophagy |
Amini Acids |
activate |
inhibit |
RagA/B (GTP-bound) |
activate |
inhibit |
Ragulator |
activate |
inhibit |
v-ATPase |
activate |
inhibit |
GATOR1 |
inhibit |
activate |
GATOR2 |
activate |
inhibit |
Sestrins |
inhibit |
activate |
Growth factor/Insulin |
activate |
inhibit |
TSC2 (TSC-TBC) |
inhibit |
activate |
Rheb (GTP-bound) |
activate |
inhibit |
NO |
activate |
inhibit |
JNK1 |
No Effect |
activate |
Beclin |
No Effect |
activate |
IKKb |
inhibit |
activate |
ammonia |
No Effect |
activate |
aKG |
activate |
inhibit |
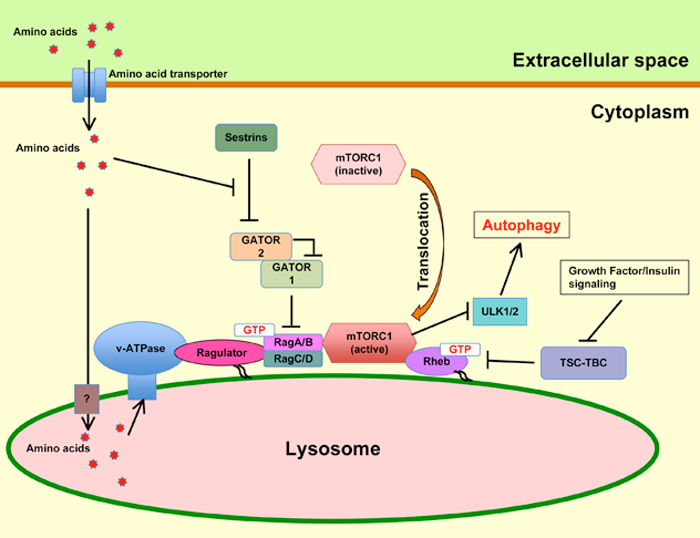
Figure 1. Regulation of mTORC1 and autophagy by amino acids. Amino acids are sensed by v-ATPase in the lysosomal lumen. v-ATPase and Ragulator change their conformation, resulting in the conversion of RagA/B into the GTP-bound state by the guanine nucleotide exchange factor (GEF) activity of Ragulator. The active GTP-bound RagA/B recruits mTORC1 to the lysosome, where Rheb activates mTORC1. Activated mTORC1 blocks autophagy. The mechanism involved in the transport of amino acids to the lysosomal lumen remains unclear. In the cytoplasm, the GTPase-activating protein (GAP) activity of GATOR1 converts RagA/B into the GDP-bound state. GATOR2 inhibits the GAP activity of GATOR1 and Sestrins inhibit GATOR2. The inhibitory effect of Sestrins on GATOR2 is blocked by amino acids and the amino acids then activate RagA/B. In low amino acid conditions, RagA/B is converted into the GDP-bound state, which cannot bind mTORC1. The inactive mTORC1 is unable to inhibit ULK1/2, thereby leading to the induction of autophagy.
Recruitment of mTORC1 to the lysosome by amino acids
mTORC1 is recruited to the lysosome surface in amino acid-rich conditions. Rag guanosine triphosphatase (GTPase) heterodimers found on the lysosomes are the key players in the translocation of mTORC1 to the lysosome membrane by amino acids. In mammals, four Rag GTPase have been found, namely RagA, RagB, RagC, and RagD [53,54]. Unlike other small GTPases, Rag GTPases have no lipid anchor and they form a heterodimer that comprises RagA or RagB with either RagC or RagD. The GTP or GDP states in Rags are regulated by amino acids. RagA/B binds to GDP and RagC/D binds to GTP in amino acid-depleted conditions, whereas RagA/B binds to GTP and RagC/D binds to GDP in amino acid-rich conditions, the latter state being the active state of the Rag heterodimer. The active Rag heterodimer binds with Raptor in mTORC1, thereby leading to the localization of mTROC1 to the lysosome membrane [53]. Rag GTPases lack a lipid anchor; hence, the Rag heterodimer is localized to the lysosomal membrane by binding to “Ragulator”, a guanine nucleotide exchange factor (GEF) and a lysosomal protein complex, which comprises five proteins, namely p18, p14, MP1, C7orf5, and HBXIP [55,56]. The binding of Ragulator to the lysosomes is possibly mediated by p18, which possesses myristoylation and palmitoylation sites on its N-terminal side [57]. Moreover, Ragulator binds to vacuolar-type H+ ATPase (v-ATPase), which is an ATP-dependent proton pump on the lysosome. The v-ATPase is a multiprotein complex, which comprises V0 (membrane-bound complex) and V1 (cytosolic complex), and it serves as a proton pump to acidify lysosomes [58]. In amino acid-rich conditions, the amino acids inside the lysosome affect v-ATPase and weaken the link between Ragulator and the V1 subunit of v-ATPase, thereby leading to changes in the state of Ragulator. Next, Ragulator converts GDP-bound RagA/B into GTP-bound RagA/B via its GEF activity. The GTP-bound RagA/B recruits mTORC1 to the lysosomal membrane [59]. RagA/B is also regulated by the GATOR complex, which comprises two subcomplexes: GATOR1 and GATOR2. GATOR1 is the GTPase-activating protein (GAP) that changes GTP-bound RagA/B into GDP-bound RagA/B and inhibits the binding between RagA/B and mTORC1. GATOR1 is a protein complex, which comprises DEP domain-containing protein 5 (DEPDC5), nitrogen permease regulator-like2 (NPRL2), and NPRL3. GATOR1 is negatively regulated by GATOR2, which comprises Mios, WD repeat-containing protein 24 (WDR24), WDR59, SEC 13 homologue-like 1 (Seh1L), and secretory 13 (Sec13). In GATOR2, Mios is necessary for the activation of mTORC1 by amino acids [60]. Therefore, GATOR1 is a negative regulator of mTORC1 and a positive regulator of autophagy. On the other hand, GATOR2 is a positive regulator of mTORC1 and a negative regulator of autophagy. Recently, it was demonstrated that Sestrins (Sestrin1/2/3) interact with GATOR2 and are necessary for the complete inhibition of mTORC1 activity in amino acid-depleted conditions [61,62]. These results indicate that GATOR1 becomes active due to the negative effect of Sestrins on GATOR2 in amino acid-depleted conditions and active GATOR1 inhibits the recruitment of mTORC1 to the lysosome, thereby leading to autophagy. Although Sestrins regulate mTORC1, additional research is needed to clarify the amino acid sensing pathway upstream of Sestrins.
Activation of mTORC1 on the lysosome
mTORC1 is activated by GTP-loaded Rheb on the lysosome membrane [63]. Rheb is a small GTPase and a membrane-binding protein, and GTP-loaded Rheb is the active form. Rheb is negatively regulated by the TSC–TBC complex, which comprises TSC1, TSC2, and Tre2-Bub2-Cdc16 1 domain family member 7 (TBC1D7). TSC2 has GAP activity for Rheb and inhibits Rheb by changing GTP to GDP. The PI3K–Akt pathway, which is related to growth factors or insulin, phosphorylates and inhibits the TSC–TBC complex, thereby leading to the activation of mTORC1 by GTP-loaded Rheb [64].
Amino acids activate RagA/B and recruit mTORC1 to the lysosome. The recruited mTORC1 is activated by interacting with GTP-loaded Rheb on the lysosome membrane surface. Thus, both amino acids and the PI3K–Akt pathway involving growth factors are considered to be necessary for the activation of mTORC1, which inhibits the autophagy pathway.
Recent studies of mTORC1 have shown that mTORC1 is a crucial factor related to the sensing and signaling of amino acids in the regulation of autophagy pathway. These include the discovery of mTORC1 regulators, such as Rag complex, Ragulator, GATOR complex, and Sestrins.
However, the upstream signal transduction pathway that regulates Sestrins and the detailed mechanisms related to the regulation of v-ATPase by amino acids remain unclear. New technological developments may help to understand the detailed mechanism involved in the sensing and signaling of amino acids to control mTORC1 and autophagy.
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10: 458-467. [Crossref]
- Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814-822. [Crossref]
- Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24: 24-41. [Crossref]
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147: 728-741. [Crossref]
- Shen HM, Mizushima N (2014) At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy. Trends Biochem Sci 39: 61-71. [Crossref]
- Cheng Y, Ren X, Hait WN, Yang JM (2013) Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev 65: 1162-1197. [Crossref]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451: 1069-1075. [Crossref]
- Mortimore GE, Schworer CM (1977) Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature 270: 174-176. [Crossref]
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, et al. (1998) Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484-14494. [Crossref]
- Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, et al. (2015) Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 347: 194-198. [Crossref]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, et al. (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136: 521-534. [Crossref]
- Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, et al. (2015) Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347: 188-194. [Crossref]
- Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40: 280-293. [Crossref]
- Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149: 274-293. [Crossref]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, et al. (2003) GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11: 895-904. [Crossref]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, et al. (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873-886. [Crossref]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316-323. [Crossref]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, et al. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177-189. [Crossref]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163-175. [Crossref]
- Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, et al. (2010) Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem 285: 20109-20116. [Crossref]
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, et al. (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20: 1981-1991. [Crossref]
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, et al. (2008) FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181: 497-510. [Crossref]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720-5728. [Crossref]
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, et al. (2004) LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci 117: 2805-2812. [Crossref]
- Tanida I, Komatsu M, Ueno T, Kominami E (2003) GATE-16 and GABARAP are authentic modifiers mediated by Apg7 and Apg3. Biochem Biophys Res Commun 300: 637-644. [Crossref]
- Wild P, McEwan DG, Dikic I (2014) The LC3 interactome at a glance. J Cell Sci 127: 3-9. [Crossref]
- Xin Y, Yu L, Chen Z, Zheng L, Fu Q, et al. (2001) Cloning, expression patterns, and chromosome localization of three human and two mouse homologues of GABA(A) receptor-associated protein. Genomics 74: 408-413. [Crossref]
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, et al. (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685-701. [Crossref]
- Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, et al. (2009) A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11: 1433-1437. [Crossref]
- Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, et al. (2010) Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656-667. [Crossref]
- Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC (2010) Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 12: 747-757. [Crossref]
- Rubinsztein DC, Shpilka T, Elazar Z (2012) Mechanisms of autophagosome biogenesis. Curr Biol 22: R29-34. [Crossref]
- Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC (2013) Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154: 1285-1299. [Crossref]
- Gutierrez MG, Munafó DB, Berón W, Colombo MI (2004) Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 117: 2687-2697. [Crossref]
- Jäger S, Bucci2021 Copyright OAT. All rights reserval. (2004) Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117: 4837-4848. [Crossref]
- Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, et al. (2014) The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell 25: 1327-1337. [Crossref]
- Liang C, Lee JS, Inn KS, Gack MU, Li Q, et al. (2008) Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol 10: 776-787. [Crossref]
- Atlashkin V, Kreykenbohm V, Eskelinen EL, Wenzel D, Fayyazi A, et al. (2003) Deletion of the SNARE vti1b in mice results in the loss of a single SNARE partner, syntaxin 8. Mol Cell Biol 23: 5198-5207. [Crossref]
- Fraldi A, Annunziata F, Lombardi A, Kaiser HJ, Medina DL, et al. (2010) Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J 29: 3607-3620. [Crossref]
- Itakura E, Kishi-Itakura C, Mizushima N (2012) The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151: 1256-1269. [Crossref]
- Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, et al. (2015) Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol Cell 57: 55-68. [Crossref]
- Kim YM, Jung CH, Seo M, Kim EK, Park JM, et al. (2015) mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol Cell 57: 207-218. [Crossref]
- Hediger MA, Clémençon B, Burrier RE, Bruford EA (2013) The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 34: 95-107. [Crossref]
- Chen R, Zou Y, Mao D, Sun D, Gao G, et al. (2014) The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J Cell Biol 206: 173-182. [Crossref]
- Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, et al. (2011) Complex inhibitory effects of nitric oxide on autophagy. Mol Cell 43: 19-32. [Crossref]
- Le Plénier S, Walrand S, Noirt R, Cynober L, Moinard C (2012) Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway? Amino Acids 43: 1171-1178. [Crossref]
- Cheong H, Lindsten T, Wu J, Lu C, Thompson CB (2011) Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A 108: 11121-11126. [Crossref]
- Eng CH, Yu K, Lucas J, White E, Abraham RT (2010) Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal 3: ra31. [Crossref]
- Harder LM, Bunkenborg J, Andersen JS (2014) Inducing autophagy: a comparative phosphoproteomic study of the cellular response to ammonia and rapamycin. Autophagy 10: 339-355. [Crossref]
- Durán RV, MacKenzie ED, Boulahbel H, Frezza C, Heiserich L, et al. (2013) HIF-independent role of prolyl hydroxylases in the cellular response to amino acids. Oncogene 32: 4549-4556. [Crossref]
- Durán RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, et al. (2012) Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 47: 349-358. [Crossref]
- Schriever SC, Deutsch MJ, Adamski J, Roscher AA, Ensenauer R (2013) Cellular signaling of amino acids towards mTORC1 activation in impaired human leucine catabolism. J Nutr Biochem 24: 824-831. [Crossref]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, et al. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496-1501. [Crossref]
- Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T (2001) Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem 276: 7246-7257. [Crossref]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196-1208. [Crossref]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, et al. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290-303. [Crossref]
- Nada S, Hondo A, Kasai A, Koike M, Saito K, et al. (2009) The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J 28: 477-489. [Crossref]
- Marshansky V, Rubinstein JL2, Grüber G3 (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837: 857-879. [Crossref]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, et al. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334: 678-683. [Crossref]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, et al. (2013) A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100-1106. [Crossref]
- Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, et al. (2014) The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9: 1-8. [Crossref]
- Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, et al. (2014) Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep 9: 1281-1291. [Crossref]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, et al. (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25: 903-915. [Crossref]
- Dibble CC, Elis W, Menon S, Qin W, Klekota J, et al. (2012) TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 47: 535-546. [Crossref]

