Exposure to high sugar diet (HSD) serves as an experimental model of insulin resistance (IR) and type 2 diabetes (T2D) in mammals and insects. Peripheral IR induced by HSD delays emergence of pupae from larvae and decreases body weight of Drosophila imago. Understanding of mechanisms of IR/T2D is essential for refining T2D prevention and treatment strategies. Dysregulation of tryptophan (TRP) – kynurenine (KYN) pathway was suggested as one of the mechanisms of IR development. Rate-limiting enzyme of TRP – KYN pathway in Drosophila is TRP 2,3-dioxygenase (TDO), an evolutionary conserved ortholog of human TDO. In insects TDO is encoded by vermilion gene. TDO is not active in vermilion mutants. In order to evaluate the possible impact of deficient formation of KYN from TRP on the inducement of IR by HSD, we compared the effect of HSD in wild type (Oregon) and vermilion mutants of Drosophila melanogaster by assessing the time of white pupae emergence from larva and body weight of imago. Delay of emergence of pupae from larvae induced by high sucrose diet was less pronounced in vermilion (1.4 days) than in Oregon flies (3.3 days) in comparison with flies maintained on standard diet. Exposure to high sucrose diet decreased body weight of Oregon (but not vermilion) imago. Attenuation of high sucrose diet–induced IR/T2D in vermilion flies might depend on deficiency of TRP – KYN pathway. Besides IR/T2D, HSD induces obesity in Drosophila. Future studies of HSD-induced obesity and IR/T2D in TDO deficient vermilion mutants of Drosophila might help to understand the mechanisms of high association between IR/T2D and obesity. Modulation of TRP – KYN metabolism might be utilized for prevention and treatment of IR/T2D
type 2 diabetes, drosophila, high sugar diet, insulin resistance, kynurenine, tryptophan, obesity
About 344 million people were diagnosed with type 2 diabetes (T2D) worldwide in 2013. T2D is the 8th leading cause of death in the world [1]. In the US about 16 million people have impaired glucose tolerance (pre-diabetes), a high-risk state for T2D: up to 70% of individuals with prediabetes eventually develop T2D [2]. Prediabetes is associated with presence of insulin resistance (IR) before T2D could be diagnosed. Understanding of mechanisms of IR, a hallmark of T2D, is essential for developing T2D prevention and treatment strategies. Dysregulation of kynurenine (KYN) pathway of tryptophan (TRP) metabolism (KP) was suggested as one of the mechanisms of development of IR and T2D [3-7]. Recently reported elevation of plasma levels of major derivatives of KP in T2D and a strong correlation between dysregulation of KP and severity of IR might further support the suggestion of KP involvement in mechanisms of IR/T2D [8,9]. Exposure to high sugar diet (HSD) induces experimental model of IR/T2D in mammals [10] and insects [11]. HSD causes metabolic dysfunction, including hyperglycemia, hyperinsulinemia, and IR in Drosophila [12]. There are four distinct stages in the life of Drosophila melanogaster: egg, larva, pupa, and imago (adult). Peripheral IR induced by HSD delays emergence of pupae from larvae and decreased body weight of imago [11-13]. Drosophila model allows for further studies of KP involvement in mechanisms of IR/T2D due to availability of natural mutants with deficient KP. Rate-limiting enzyme of KP in Drosophila is TRP 2,3-dioxygenase (TDO), an evolutionary conserved ortholog of human TDO, that is encoded by vermilion gene. TDO is inactive in vermilion mutants of Drosophila melanogaster [14]. In order to evaluate the possible impact of deficient formation of KYN from TRP on the inducement of IR by HSD, we compared the effect of HSD on development of IR in vermilion mutant and wild type (Oregon) Drosophila melanogaster.
Wild-type stock Oregon and vermilion mutants of Drosophila melanogaster from the collection of V.N. Karazin Kharkiv National University were maintained at 23°C in a 12:12 light: dark period on a standard nutrition medium consisting of sugar, yeast, agar and semolina. Eggs were obtained from synchronized egg laying from fertilized flies. Sucrose (0.67M) was added to nutrition medium before eggs laying. Emerging time was taken as the period from the time of synchronized egg laying to the time of larvae emergency into white pupae as described elsewhere [15]. Imago (males) were weighed in groups of 10 flies, with a precise balance. The study was carried out between April and July 2014.
Statistics data from three replicated experiments was used for the statistical analyses. The data were expressed as mean ± standard deviation (hours for pupae emergence and mg for body weight). Differences between experimental groups were evaluated by Mann Whitney, two-tailed test.
Pupae emergence from larva
Emergence time of pupae from larva of Oregon flies maintained on standard nutrition medium was 16% shorter than emergence time of vermilion flies (Table 1).
Table 1. Effect of high glucose diet on time of pupae emergency from larvae and body weight in vermilion and Oregon flies
Genotype and treatment |
Emergence of pupae (hrs) |
Weight of imago (mg) |
Oregon (control) |
151.2 ± 19.2 (n=150) |
0.99 ± 0.004 |
Oregon + High Sucrose Diet |
232.5 ± 23.1 (n=150)* |
0.62 ± 0.003* |
Vermilion (control) |
176.5 ± 27.6 (n=360)** |
0.93 ± 0.004 |
Vermilion + High Sucrose Diet |
208.9 ± 22.2 (n=360)*# |
1.07 ± 0.003 |
Mann-Whitney two tailed test
*p<0.0001 in comparison with respective controls
**p<0.0001 in comparison with Oregon controls
#p<0.0001 in comparison with Oregon + high sucrose diet
High sucrose diet delayed pupae emergence from larva of wild type flies (Oregon) in comparison with flies maintained on standard nutrition medium by 3.3 days.
In vermilion mutants high sucrose diet delayed pupae emergence from larvae by 1.4 days in comparison with flies maintained on standard nutrition medium.
High sucrose diet-induced delay of pupae emergence from larvae was shorter in vermilion than in Oregon flies (Table 1).
Body weight of imago
There were no differences in body weight of male imago Oregon and vermilion flies maintained on standard nutrition medium (Table 1).
High sucrose diet decreased body weight of wild type flies (Oregon) imago by 37%.
In vermilion mutants high sucrose diet did not affect body weight of imago.
The main finding of the present study is that high sucrose diet induced three times shorter delay of pupae emergence from larvae and did not decrease body weight of imago in vermilion flies in comparison with wild type flies. We are not aware of studies of the effect of HSD on preimaginal stages of TDO deficient vermilion flies. Considering that HSD-induced delayed emergence of pupae and decreased body weight is caused by IR [11-13], our data suggest that inducement of IR by high sucrose diet is attenuated in vermilion mutants in comparison with wild type Drosophila.
Attenuation of HSD-induced IR in vermilion flies most likely depends on deficiency of TDO. In Drosophila, TDO-regulated KYN formation from TRP begins at the end of the third larval instar in the cells of the anterior region of fat body (analog of liver and fat tissues in humans) [14].
Transition from larva to pupae in Drosophila is triggered by a steroid hormone, ecdysone [16]. Considering that insulin activates ecdysone formation, HSD-induced resistance to insulin effects might impair ecdysone formation and, consequently, delay pupariation [16]. Attenuation of IR development in KP deficient vermilion mutants might attenuate HSD-induced delay of transition from larvae to pupae by facilitation of ecdysone formation.
It is noteworthy that HSD induces IR/T2D in mammals as well. Immediate metabolic response to HSD is an increase of glucose in insects [12] and in mammals [10]. Hyperglycemia activates hypothalamic-pituitary-adrenal axis and increases cortisol secretion in rat model of T2D [17] and in T2D patients [18]. Cortisol activates TDO, rate-limiting enzyme of KP, that converts TRP into a number of biologically active metabolites, including KYN, a precursor of kynurenic acid (KYNA) and xanthurenic acid (XA) [19]. Although end products of KYN pathway of TRP metabolism are different in humans (NAD+) and flies (ommochrome, brown eye pigment) [20], both species produce KYNA and XA [21]. Clinical and experimental studies suggested that XA and KYNA exert diabetogenic effects [22-26]. Therefore, inducement of IR by HSD might be mediated via increased production of diabetogenic KYN derivatives, XA and KYNA, in mammals (Figure 1).
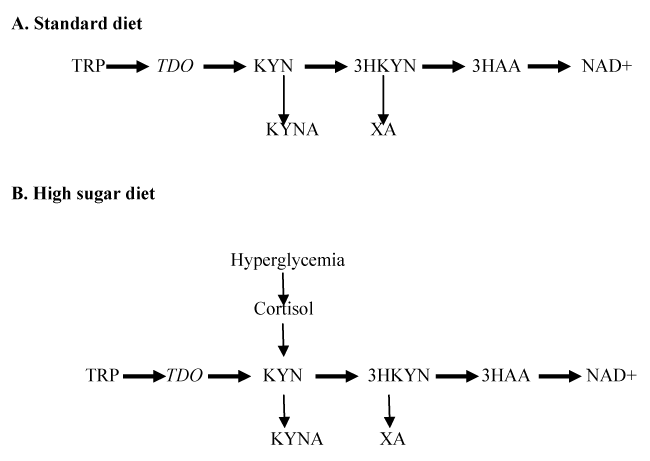
Figure 1. Hypothetical effect of high sugar diet on TRP – KYN pathway
Abbreviations: TRP: Tryptophan; TDO: TRP 2,3-dioxygenase; KYN: kynurenine; 3-HKYN: 3-hydroxyKYN; 3-HAA: 3-hydroxyanthranilic acid; KYNA: kynurenic acid; XA: xanthurenic acid
Currently, mechanism of HSD-induced IR is considered to depend on Lipocalin Neural Lazarillo, a secreted protein homologous to the Retinol-Binding Protein 4 (RBP) involved in the onset of T2D in human [27,28] and mice [29]. It is noteworthy that linear correlation was found between the postprandial increases of TRP and RBP in morbidly obese subjects [27].
Obesity is highly associated with T2D [30]. The mechanisms of such association are not clear. Dysregulation of KP in obesity was suggested [3-7] and supported by clinical and experimental data [31-34]. It is noteworthy that in Drosophila HSD induces not only IR/T2D but obesity as well [11]. Future studies of HSD-induced obesity in vermilion mutants might provide better understanding of the role of KP in mechanisms of obesity and T2D.
Present data suggest disturbances of KP as one of the mechanisms mediating HSD-induced IR/T2D. KP might be a new target for prevention and treatment of IR and T2D.
GF Oxenkrug is a recipient of MH104810. Paul Summergrad is a non-promotional speaker for CME outfitters, Inc., and consultant and non-promotional speaker for Pri-med, Inc.
- Tao Z, Shi A, Zhao J (2015) Epidemiological Perspectives of Diabetes. Cell Biochem Biophys. [Crossref]
- Herder C, Nuotio ML, Shah S, Blankenberg S, Brunner EJ, et al. (2014) Genetic determinants of circulating interleukin-1 receptor antagonist levels and their association with glycemic traits. Diabetes 63: 4343-4359. [Crossref]
- Connick JH, Stone TW (1985) The role of kynurenines in diabetes mellitus. Med Hypotheses 18: 371-376. [Crossref]
- Oxenkrug GF (2010) Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci 1199: 1-14. [Crossref]
- Oxenkrug G (2013) Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 48: 294-301. [Crossref]
- Oxenkrug G (2015) 3-hydroxykynureninc acid and type 2 diabetes: implications for aging, obesity, depression, Parkinson’s disease and schizophrenia. In: A. Engin, A.B. Engin (Eds.), Tryptophan Metabolism: Implications for Biological Processes, Health and Diseases, Molecular and Integrative Toxicology: 173-195.
- Polyzos KA, Ketelhuth DF (2015) The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie 35: 128-136. [Crossref]
- Oxenkrug GF (2015) Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol Neurobiol. [Crossref]
- Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, et al. (2015) Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol 35: 455-462. [Crossref]
- Félétou M, Boulanger M, Staczek J, Broux O, Duhault J (2003) Fructose diet and VEGF-induced plasma extravasation in hamster cheek pouch. Acta Pharmacol Sin 24: 207-211. [Crossref]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, et al. (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4: 842-849. [Crossref]
2021 Copyright OAT. All rights reserv
- Pasco MY, Léopold P (2012) High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One 7: e36583. [Crossref]
- Rovenko BM, Perkhulyn NV, Gospodaryov DV, Sanz A, Lushchak OV, et al. (2015) High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comp Biochem Physiol A Mol Integr Physiol 180: 75-85. [Crossref]
- Rizki TM, Rizki RM (1964) Factors affecting the intracellular synthesis of kynurenine. J Cell Biol 21: 27-33. [Crossref]
- Navrotskaya V, Oxenkrug G, Vorobyova L, Sharma H, Muresanu D, et al. (2014) Cerebrolysin Accelerates Metamorphosis and Attenuates Aging-Accelerating Effect of High Temperature in Drosophila Melanogaster. Am J Neuroprot Neuroregen 6: 65-68. [Crossref]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, et al. (2005) Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310: 667-670. [Crossref]
- Elahi-Moghaddam Z, Behnam-Rassouli M, Mahdavi-Shahri N, Hajinejad-Boshroue R, Khajouee E (2013) Comparative study on the effects of type 1 and type 2 diabetes on structural changes and hormonal output of the adrenal cortex in male Wistar rats. J Diabetes Metab Disord 12: 9. [Crossref]
- Chiodini I, Torlontano M, Scillitani A, Arosio M, Bacci S, et al. (2005) Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur J Endocrinol 153: 837-844. [Crossref]
- Oxenkrug GF (2007) Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci 1122:35-49. [Crossref]
- van der Goot AT, Nollen EA (2013) Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends Mol Med 19: 336-344. [Crossref]
- Real MD, Ferré J (1990) Biosynthesis of xanthurenic acid 8-O-beta-D-glucoside in Drosophila. Characterization of the xanthurenic acid:UDP-glucosyltransferase activity. J Biol Chem 265: 7407-7412. [Crossref]
- Ikeda S, Kotake Y (1986) Urinary excretion of xanthurenic acid and zinc in diabetes: (3). Occurrence of xanthurenic acid-Zn2+ complex in urine of diabetic patients and of experimentally-diabetic rats. Ital J Biochem 35: 232-241. [Crossref]
- Kotake Y, Ueda T, Mori T, Igaki S, Hattori M (1975) Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA). Acta Vitaminol Enzymol 29: 236-239. [Crossref]
- Oxenkrug GF, Turski WA, Zgrajka W, Weinstock JV, Summergrad P (2013) Tryptophan-kynurenine metabolism and insulin resistance in hepatitis C patients. Hepat Res Treat 2013: 149247. [Crossref]
- Patterson AD, Bonzo JA, Li F, Krausz KW, Eichler GS, et al. (2011) Metabolomics reveals attenuation of the SLC6A20 kidney transporter in nonhuman primate and mouse models of type 2 diabetes mellitus. J Biol Chem 286: 19511-19522. [Crossref]
- Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, et al. (2010) Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem 285: 21913-21921. [Crossref]
- Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, et al. (2007) Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 53: 34-41. [Crossref]
- Kihlberg R, Bark S, Hallberg D (1982) An oral amino acid loading test before and after intestinal bypass operation for morbid obesity. Acta Chir Scand 148: 73-86. [Crossref]
- van Dam RM, Hu FB (2007) Lipocalins and insulin resistance: etiological role of retinol-binding protein 4 and lipocalin-2? Clin Chem 53: 5-7. [Crossref]
- Gallagher EJ, LeRoith D (2015) Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev 95: 727-748. [Crossref]
- Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, et al. (2014) Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 22: 195-201. [Crossref]
- Wolowczuk I, Hennart B, Leloire A, Bessede A, Soichot M, et al. (2012) Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol 303: R135-R143. [Crossref]
- Brandacher G, Hoeller E, Fuchs D, Weiss HG (2007) Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab 8: 289-295. [Crossref]
- Watts SW, Shaw S, Burnett R, Dorrance AM (2011) Indoleamine 2,3-diooxygenase in periaortic fat: mechanisms of inhibition of contraction. Am J Physiol Heart Circ Physiol 301: H1236-H1247. [Crossref]

