To investigate roles of short peptides in gastroschisis (GS), we comprehensively analyzed peptides in amniotic fluid (AF), creating a fetal lamb model of GS. We created GS in 4 fetal lambs at 60 days of gestation. Three GS and 4 normal fetuses were delivered at term (145 days) by cesarean section, when AF samples were collected. Short peptides in the AF samples were detected and identified by mass spectrometry. One of the identified peptides was synthesized and it’s functions were investigated. In total, 77 peptide peaks were detected in the AF samples. Of these, 12 peptides showed significantly different intensity between the GS and control groups. Three of the 12 peptides were identified. One of the identified peptides with high intensity in the GS group was amino acids (AA) 135-185 of lamb annexin 7 (ANX7). A synthesized peptide for AA168-211 of human ANX7, which corresponded to AA135-185 of lamb ANX7, decreased anti-inflammatory cytokine secretion from mesothelial cells by an cytokine array study. We report a unique AF peptide profile in a GS model. One of the peptides increased in GS was suggested to possess pro-inflammatory potential. These peptides would be related to the pathophysiology of GS.
gastroschisis, fetal surgery, peptide, amniotic fluid, cytokine, sheep
Gastroschisis (GS) is a congenital disease characterized by an abdominal wall defect through which bowel protrudes. The defect is almost always located to the right of umbilicus [1]. The antenatal diagnosis of GS is usually made by ultrasonographic observation of bowel loops floating in amniotic fluid (AF). The frequency of GS has increased over the last few decades, increasing from 0.6/10,000 live births in 1980-1984 to 2.33/10,000 live births in 2000-2002 [2]. GS occurs sporadically, appearing to have no genetic links. The pathogenesis of GS remains unclear, although it is possibly associated with a rupture of the physiological umbilical hernia and the regression of the umbilical vein [3]. Recently, the prognosis of GS has been improved significantly by the use of a Silo during closure [4] and the ready availability of total parenteral nutrition [5]. However, GS associated with bowel edema, intrauterine growth retardation, oligohydramnios, and/or bowel atresia still has a poor prognosis [6]. In many infants with GS the protruding bowel is covered by a fibrinous “peel”. There may be ischemic changes and dilatation macroscopically. Histologically, there is thickening and edema of the muscularis mucosae and serosal layers [7].
There are several theories put forward to explain the bowel inflammation and the development of “peel” in GS, with exposure to AF being most commonly implicated [8]. The level of ferritin, a marker of chronic inflammation and the levels of interleukin (IL)-6 and IL-8, proinflammatory cytokines, have been reported to be increased significantly in AF of patients with GS [7]. Many inflammatory cells such as macrophages are also detected in AF of patients with GS [7]. In this context, it would be of great interest to determine whether there are bioactive molecules in AF that may affect the protruding bowel. Possibilities include proteins like IL-6 and IL-8 as mentioned above. Even though protein components in AF have been comprehensively analyzed in humans in vivo [9], AF proteins from patients with GS has not been analyzed, to our knowledge.
Another important type of molecule would be short peptides. Short peptides are thought to be generated by physiological cleavage of precursor proteins and by pathological degradation of various proteins. The former includes essential bioactive peptides like substance P [10] and defensin [11]. As examples of the pathological degradation of proteins, the level of C3f-des-arginine, a degraded product of C3 was reported to be increased in systemic sclerosis and C3f-des-arginine also enhances proliferation of vascular endothelial cells [12]. The level of AC13, a degraded peptide of apolipoprotein A, was reported to be increased in microscopic polyangiitis. Also, AC13 enhances production of IL-6 and IL-8 from endothelial cells [13]. However, such degradation-mediated peptides have been poorly understood until now. To our knowledge, there has been no report on degradation-mediated peptides in AF in GS, we thus have tried to elucidate profiles of short peptides using a fetal lamb model of GS.
Creation of a fetal lamb GS model and collection of AF samples
Lambs at 60 days of gestation were subjected to surgical operation to create experimental GS. The preoperative management and anesthetic techniques have been reported previously [14,15]. Under general anesthesia, the uterus was exposed through a left flank incision, and the fetuses (n=4) were exposed through a transverse hysterotomy. An incision was made in the left upper part of the fetal abdominal wall and the bowel was gently delivered through the incision. The fetuses were then returned to uterus, with the hysterotomy being closed in 2 layers with 3-0 Biosyn. The ewe’s abdominal wall was closed in layers with Biosyn or Polysorb (US Surgical, Tyco Healthcare, Tokyo, Japan). AF samples from normal lambs (n=4) were used as controls.
The fetuses were delivered at term (145 days) by cesarean section, when approximately 4 ml of AF samples were collected by a syringe and immediately cooled by dry ice and stored at -80ºC.
This study was approval by the ethics committee of School of Medicine and Health Sciences, University of Otago, Wellington Animal Ethics Committee (Wellington, New Zealand Approval Number 2-12).
Detection and identification of AF peptides
Peptides in the AF samples were purified using weak cation exchange (MB-WCX; Bruker Daltonics, Ettliongen, Germany) as described previously [16]. Then mass-to-charge ratios (m/z) and ion intensity of the peptides was measured by matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry (MALDI-TOF/MS; Bruker Daltonics).
For the identification of peptides of interest, MS/MS data of the peptides obtained by ESI-ion trap mass spectrometry was subjected to the searching program of MASCOT MS/MS ion search (Matrix Science, London, UK) against the Sheep Gene Index. Peptide identification was accepted when MASCOT search results delivered significant molecular weight search (MOWSE) scores (p< 0.05).
Western blot analysis
For detection of lamb annexin A7 (ANX7), rabbit anti-human ANX7 polyclonal antibodies (Lifespan Biosciences, WA, USA) were used, since no antibody to lamb ANX7 was available. In a preliminary experiment, we confirmed that the antibody reacted to lamb ANX7 (data not shown). Proteins extracted from AF samples were separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then were transferred onto a poly vinylidene fluoride (PVDF) membrane. After blocking for 1 hour in a blocking buffer (TBS containing 2% ECL prime blocking agent (GE healthcare) and 0.1% Tween-20), the membrane was reacted to the anti-ANX7 antibodies at a dilution of 1:500 for 1 hour. After washing, bound antibodies were reacted with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (Dako, Glostrup, Denmark) and the substrate of HRP using ImmunoStar LD (Wako Co., Osaka, Japan). Finally, immunoreactive bands were detected using an image analyzer (LAS-3000 Versatile Imaging System Fujifilm, Tokyo, Japan).
A peptide corresponding to AA168-211 of human annexin A7 was synthesized and named as hA7 (168-211) The region of hA7 (168-211) is a homologous part of AA135-185 of lamb annexin7.
Human adult mesothelial (HAM) cells were purchased from Zen-bio (CA, USA). HAM cells, seeded on type1 collagen-coated dishes with a diameter of 10 cm, were cultured at 37° in normoxic air with 5% CO2 in Mesothelial Cell Growth Medium (ZEN-bio, CA, USA). After HAM cells were cultured in a starvation medium (Medium 199, Life Technologies, CA, US), 2% Penicillin G(10000 unit/ml)/Storeptomycin (10 mg/ml) (SIGMA, UK), 10% charcoal dextran-treated Fetal bovine serum (Thermo SCIENTIFIC, MA, US)) for 24 hours, the cells were further incubated with or without 400n mol/ml of hA7 (168-211) for 48 hours. Finally, supernatant of the cell culture was collected and subjected to cytokine array analysis using a commercially available kit of Human Cytokine Array Panel A (R&D systems, Inc, MN, USA), which contained 36 different cytokines (complement component C5a, CD40 ligand, G-CSF, CXCL1/GRO α, CCL1/I-309, sICAM-1, IFN-γ, IL-1 α, IL-1 β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 p70, IL-13, IL-16, IL-17, IL-17E, IL-23, IL-27, IL-32 α, CXCL10/IP-10, CXCL11/I-TAC, CCL2/MCP-1, MIF, CCL3/MIP-1 alpha, CCL4/MIP-1 β, CCL5/RANTES, CXCL12/SDF-1, Serpin E1/PAI-1, TNF-α, TREM-1)
Statistical analysis
Differences of the peptide ion intensity between the GS and control groups were calculated by Student’s t-test. P< 0.05 was considered to be significant.
Creation of a fetal lamb model of GS
We created experimental GS using 4 lamb fetuses at 60 days of gestation, as shown in Figure 1A. At term (145 days), 3 of the 4 fetuses were found possess GS (Table 1). A representative case is shown in Figure 1B. Just before the cesarean section AF samples were collected by puncturing the uterine wall with an 18G needle. As a control, AF samples were collected from 4 lamb fetuses of healthy mothers at term. The crown-to-rump length and the weight of the fetuses showed no significant difference between the GS and control groups, even though the mean weight was slightly heavier in the GS group (Table 1). The mean of AF protein concentration was significantly elevated in the GS group compared with the control group (Table 1).
Table 1. The profile of fetal lambs with experimental GS and control lambs
Group |
Number |
Body weight
(g) |
Crown-to-rump length
(cm) |
GS |
AF protein concentration
(mg/ml) |
GS |
1 |
3860 |
45 |
+ |
5.32 |
2 |
1890 |
37 |
+ |
4.84 |
3 |
3300 |
45 |
+ |
1.00 |
4 |
3472 |
44 |
- |
4.40 |
mean±SD |
|
3130.5 ± 859.5 |
42.8 ± 3.86 |
|
3.89 ± 1.96* |
Control |
1 |
1966 |
34 |
- |
0.39 |
2 |
1966 |
44 |
- |
0.87 |
3 |
2766 |
44 |
- |
0.25 |
4 |
2976 |
37 |
- |
0.51 |
mean±SD |
|
2418.5 ± 529.5 |
39.8 ± 5.06 |
|
0.54 ± 0.31 |
GS= gastroschisis, AF= amniotic fluid, SD= standard deviation, *= p< 0.05 (t-test).
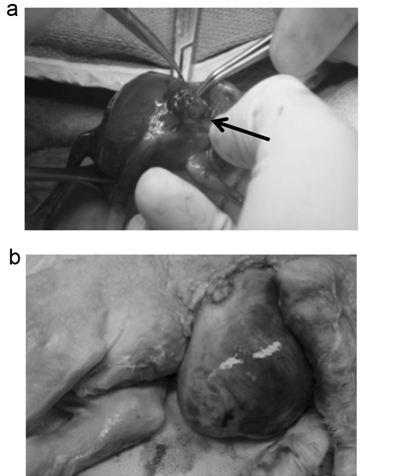
Figure 1. Creation of a fetal lamb model of GS. (a) At 60 days of gestation, fetuses were exposed through a transverse hysterotomy, then the left upper abdominal wall was incised and bowel was gently delivered through the incision (arrow). (b) The fetuses with experimental GS were delivered at term (145 days).
Detection of peptide peaks in the AF samples
We then purified peptides from each of the AF samples using MB-WCX and determined peptide profiles of the AF samples in the range of 2000-10000 m/z by MALDI-TOF-MS. In this experiment the AF sample from the GS number 4 lamb that did not posses GS was omitted. A total of 77 peptide peaks were detected in the AF samples. The peptide profiles of the individual lamb fetuses and the average peptide profiles of the GS (n=3) and control (n=4) groups are shown in Figure 2 and 3, respectively.
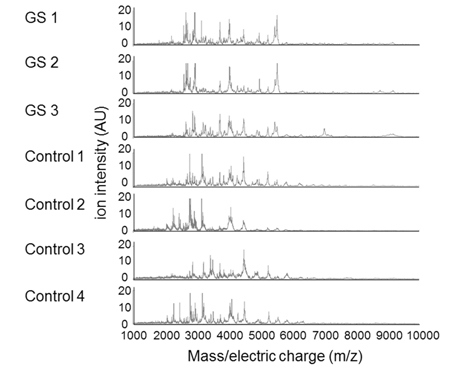
Figure 2. AF peptide peaks were detected in the GS (n=3) which except abdominal wall closure case and control (n=4) groups by MALDI-TOF/MS. m/z= mass-to-charge ratios.
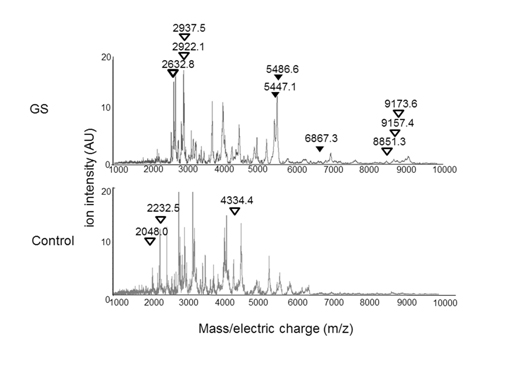
Figure 3. The average AF peptide profiles of the GS (n=3) and the control (n=4) groups.
Arrowheads indicate peptides with a significant difference between the GS and control groups (P< 0.05). Black arrowhead= peptide sequences were identified. White arrowhead= peptide sequences were not identified. m/z= mass-to-charge ratio.
Comparison of peptide profiles between the GS and control groups
We then compared the intensity of the 77 peptide peaks detected between the GS group and the controls. The intensity of 12 peptide peaks showed significant differences between the two groups (Figure 3). Specifically, 9 out of the 12 peptides showed more than 1.5-fold higher intensity in the GS group than in the control group. The remaining 3 peptides showed less than 1/1.5-fold lower intensity in the GS group than in the control group, as shown in Table 2.
Table 2. Analysis of peptide peaks between the GS and control groups
Difference (x) |
Numbers of peptide peaks |
(fold change, GS/control) |
average alone |
with statistical significance |
3.0<x |
15 |
6 |
2.0<x |
23 |
9 |
1.5<x |
31 |
9 |
1.5≤x≤1/1.5 |
24 |
0 |
x<1/1.5 |
22 |
3 |
x<1/2.0 |
13 |
2 |
x<1/3.0 |
6 |
1 |
total peptide peaks |
77 |
12 |
Identification of the peptides with significant differences between the two groups
We then tried to identify the 12 peptides by mass spectrometry and protein data base searching. As a result, we were able to identify 3 out of the 12 peptides, which were amino acid residues (AA) 135-185 of ANX7, AA163-218 of nuclear pore glycoprotein p62 (NUP62), and AA89-153 of ubiquitin fusion degradation protein 1 homolog (UFD1), as shown in Table 3.
Table 3. Identified peptides with significant differences between the GS and control groups.
Peptide
(m/z) |
Difference* |
Identified amino acid sequences
(position of amino acid) |
Theoretical
(m/z) |
Original protein |
Mascot
Score |
5486.6 |
5.54 |
GQPPYPSQPAAM TQGTQGTIRPAANFDAMRD AEVLRKAMKGF GTDEQAIIDV (135aa-185aa) |
5487.8 |
annexin A7
(ANX7) |
75 |
5447.1 |
2021 Copyright OAT. All rights reserv
4.99 |
TTTTTTTTTTTS TSTTGFSLNIKPLTPAGIPSN TAASGSAPSGPS VAAGGSGSAALT (163aa-218aa) |
5449.5 |
nuclear pore glycoprotein p62
(NUP62) |
71 |
6867.3 |
2.22 |
SGEPRASGARLRR LPLRGGVGGPHRQRS PAQRSGRARRRRPP CSPSTCSTTR SPGSSRAASPRSTA (89aa-153aa) |
6870.6 |
ubiquitin fusion degradation protein 1 homolog
(UFD1) |
65 |
m/z= mass/electric charge ratio, aa= amino acid, *= Fold change of GS/Control in intensities
Detection of intact ANX7 in AF from the fetal lamb model of GS
As above, we demonstrated the increased intensity of 12 peptides in AF of fetal lambs with GS and identified 3 out of them. Focusing on one of the 3 peptides, which was assigned to AA135-185 of lamb ANX7, we extended our study. First, we investigated whether the amount of intact ANX7 molecules was increased or not by western blotting. As a result, the intact ANX7 with its expected molecular weight (MW) of 49.9 kDa was detected both in the GS and normal AF samples (Figure 4A). The intensity of the intact ANX7 bands showed no significant difference between the GS and control lambs (p=0.432) as shown in Figure 4B. This indicates that the increase of the peptide for AA135-185 of ANX7 would be due to the increase of both production and cleavage of ANX7.
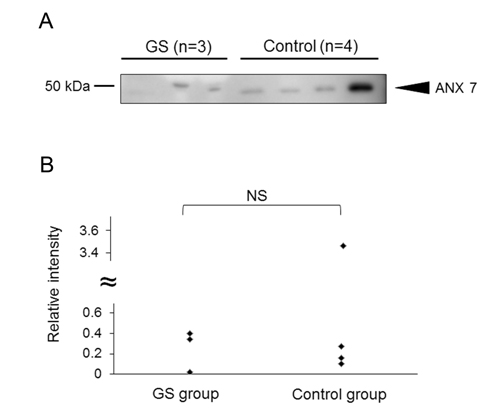
Figure 4. Detection of intact ANX7 in the AF samples from the GS and control groups. (A) Intact lamb ANX7 was detected by western blot using anti-human ANX7 polyclonal antibodies. In preliminary experiment, we confirmed that the antibody reacted to lamb ANX7. (B) The relative intensity of ANX7 was calculated by an image analyzer. The relative intensity averages of “control” were defined as 1. The intensity was compared to the GS and control groups. NS= not significant.
Effects of a human ANX7 peptide that corresponded to AA135-185 of lamb ANX7 on cytokine secretion from HAM cells
We then examined whether the region of AA135-185 lamb of ANX7 affect functions of cells. For this aim, we synthesized a peptide for AA168-211 of human ANX7 (hA7(168-211)), which corresponded to the region of AA135-185 of lamb ANX7 and examined effects of the synthesized peptide on secretion of various cytokines and soluble factors from HAM cells.
We cultured HAM cells with or without hA7 (168-211) and then the culture supernatant was applied to a cytokine array. As a result, 10 soluble factors (sICAM-1, IL-Ra, CXCL-1, IL-6, IL-8, IL-13, IL-27, MCP-1, MIF, and serpin E1) were positively detected in hA7 (168-211)-stimulated or non-stimulated panels. (Figure 5a). Among the 10 soluble factors, 4 factors (IL-1Ra, sICAM-1, IL-27 and MIF) showed more than 1.5 fold- or less than 1/1.5 fold changes. The intensity of IL-1Ra was decreased drastically by the hA7 (168-211) stimulation (0.34 fold). To lesser extent, intensity of sICAM-1, IL-27 and MIF was decreased (0.66, 0.63, and 0.66 folds, respectively). This indicates that a fragment of ANX7, hA7 (168-211), affects the profile of cytokines and soluble factors secreted from cells.
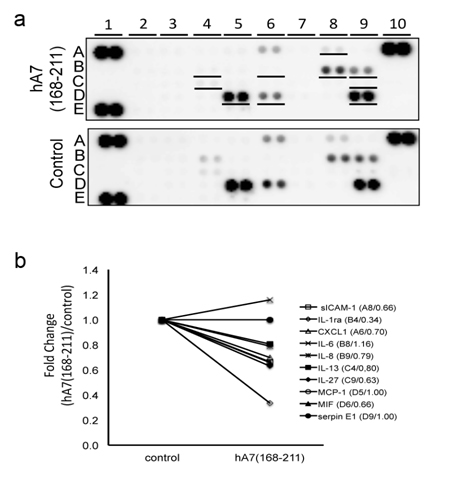
Figure 5. Effects of hA7 (168-211) on cytokine secretion from cultured mesothelial cells. (a)Human adult mesothelial cells, cultured in a starvation medium for 24 hours, were further incubated with or without hA7(168-211) for 48 hours. Then supernatant of the cell culture was collected and subjected to cytokine array analysis that contained 36 different cytokines in duplicate. The underlined spots in the upper panel indicate cytokines with detectable expression levels. A1, A10, and E1: positive control spots for the assay. E19 and E20: negative control spots. (b) Intensity of spots for each cytokine in the panels in (a) was measured. On 10 cytokines with detectable expression levels, their fold changes by the stimulation with hA7(168-211) are shown. Their names, spot location in the panels in (a), and their fold changes are described as “name (spot location/fold change).”
In this study, to clarify the pathophysiology of the “peel” found in some infants with GS, we created a fetal lamb model of GS and comprehensively analyzed peptides in AF. Until now, no report has been available on peptide profiles of AF of GS both in humans and experimental animal models. Our findings are as follows: first, 3 of the 4 GS in which GS creation was attempted, displayed GS at term. Second, the intensity of 12 out of the detected 77 peptides in AF showed significant difference between the GS and control groups. Third, three out of the 12 peptides were identified as AA135-185 of ANX7, AA163-218 of NUP62, and AA89-153 of UFD1. Fourth, the levels of the intact ANX7 showed no significant difference between the GS and control groups.
On the first point, we tried to create a GS model using 4 lamb fetuses, not all of the operated fetuses showed GS at term. This suggests that a simple incision of the abdominal wall in an early-gestation lamb fetus can heal over, unless sufficient bowel is delivered through the defect. The abdominal wall defect observed in patients with GS would involve an additional factor which maintains the abdominal wall defect and GS. Alternatively, the time to create experimental GS may be critical. Here, the experimental GS was created at 60 days of gestation as our first trial, since we successfully created an obstructive uropathy lamb model at the same days of gestation previously [17]. However, if we operated later in gestation, GS may be created more successfully. This point should be clarified by future trials.
On the second point, the intensity of 12 out of the detected 77 peptides in AF was different between the GS and control groups. This indicates that profiles of proteins contained in AF, which are the source of degraded peptides, are skewed in GS. Alternatively, profiles of proteases that actually produce degraded peptides could be altered. Considering the points that amounts of proteins in AF has been reported to be increased in patients with GS [7] and that AA135-185 of ANX7 was increased in spite of unchanged levels of the intact ANX7 in our study, both mechanisms could be involved in the aberrant degradation of proteins in AF of GS.
On the third and fourth points, we successfully identified 3 out of the 12 peptides. This identification ratio in this study was lower than is usual in our human studies. This is probably due to the fact that the size of the protein data bases in lambs was much smaller than in that in humans, which would hamper effective protein identification.
One of the parent proteins of the identified three peptides was ANX7. In humans, production of ANX7 was demonstrated in liver, kidney, spleen, and placenta [18]. Thus, ANX7 in AF detected here is thought to have originated from the lamb’s placenta. Functionally, ANX7 facilitates exocytosis upon the activation by phosphorylation in humans [19]. Accordingly, a decrease of ANX7 by siRNA was found to decrease IL-8 secretion in SW982 cells [20]. Further, an increase of both total and phosphorylated ANX7 was reported in synovial cells of patients with rheumatoid arthritis. The administration of anti-ANX7 antibodies suppressed type II collagen-induced arthritis in mice [20]. From these reports, ANX7 may promote inflammation. However, we here demonstrated that the amounts of ANX7 itself showed no significant difference between the GS and control groups. Thereby, it would be unlikely that the intact ANX7 in AF contributes to the inflammation of bowel in GS as an independent factor.
In our study, the increase of AA135-185 of ANX7 was demonstrated. In the production of this peptide derived from ANX7, certain endopeptidases that cleave ANX7 between Gly135 and Val185 should be involved. We therefore searched for such endopeptidases using a program MEROPS (http://merops.sanger.ac.uk/). However, we found no endopeptidases for production of the peptide by cleavage of ANX7 (data not shown). The mechanism for the production AA 135-185 needs to be clarified in the future.
Another interest is whether the peptide of AA135-185 of ANX7 possesses bioactivity. We previously demonstrated that AC13 which corresponded to the C-terminal 13AA of apolipoprotein A enhanced IL-6 and IL-8 secretion from endothelial cells [13] and that des-Arg-C3f, a degraded 16AA-long peptide of C3f increased production of transforming growth factor 1 in dermal microvascular endothelial cells [12]. We hypothesize that the peptide of AA135-185 of ANX7 may have functions involved in the pathophysiology of the “peel” seen in GS. Thereby, we performed the cytokine array study to elucidate functions of the peptide. We here used HAM cells and hA7 (168-211) instead of AA135-185 of lamb ANX7. It was because no lamb cell line was available and the final goal of our research was elucidation of biochemical mechanisms for GS in humans. In GS, the serous membrane derived from mesodem is exposed to AF. Thereby we selected a human mesothelial cell line of HAM cells as target cells. Among detected the 10 soluble factors, no factor showed more than1.5-fold changes and 4 factors of IL-1Ra, sICAM-1, IL-27, and MIF showed less than1/1.5-fold changes. The most decreased by the stimulation with hA7 (168-211) was IL-1Ra, an antagonist for the IL-1β receptor, which possesses anti-inflammatory activities by inhibiting the binding of IL-1β to its receptor [21]. Similarly, sICAM-1 has been thought to have protective functions from inflammation [22]. IL-27 has been reported to show anti-inflammatory activities by suppressing pro-inflammatory cytokines [23]. Thereby, the down-regulation of IL-1β, sICAM-1, and IL-27 by hA7 (168-211) would contribute to the inflammation of the bowel in GS. Of course, since the stimulation with hA7 (168-211) increased the level of MIF, an inflammatory mediator, and this cytokine array assay was rather simple screening, the contribution of hA7 (168-211) to the inflammation should be confirmed by future studies.
Parent proteins of the other two identified peptides were NUP62 and UFD1. NUP62 is an essential component of the nuclear pore complex [24]. However, roles of NUP62 and its degraded peptides in GS remain to be elucidated. UFD1 is involved in the metabolism of ubiquitinated proteins. The functions of UFD1 are largely unknown. Roles of its degraded peptides in GS are completely unknown. We plan to investigate this in the near future.
In conclusion, we have created a fetal lamb model of GS in this study. We detected 12 peptides that were increased in the AF samples the of GS model, compared to the control and identified 3peptides. A human homolog of one of the increased AF peptides, hA7 (168-211), was found to have pro-inflammatory potential. The peptides increased in the GS model would be involved in the pathophysiology of the “peel” seen in GS.
The authors have no financial conflicts of interest to disclose concerning this study.
- 1. Gabrielli S, Reece EA (1992) Gastrointestinal and genitourinary anomalies. In: Reece EA, Hob-bins JC, MAhomey MJ, Petrie RH (eds) Medicine of the fetus and the mother. JB Lippincott Companey, Philadelphia: 551-577 1. Gabrielli S, Reece EA (1992) Gastrointestinal and genitourinary anomalies. In: Reece EA, Hob-bins JC, MAhomey MJ, Petrie RH (eds) Medicine of the fetus and the mother. JB Lippincott Companey, Philadelphia: 551-577
- 2. Loane M, Dolk H, Bradbury I; EUROCAT Working Group (2007) Increasing prevalence of ga-stroschisis in Europe 1980-2002: a phenomenon restricted to younger mothers? Paediatr Perinat Epidemiol 21: 363-369. [Crossref]
- 3. Klein MD (2006) Congenital Defects of the Abdominal Wall, in: Grosfeld JL, O’Neill JA, Coran AG (eds): PEDIATRIC SURGERY. MOSBY, Philadelphia 1157-117.
- 4. Hrabovsky EE, Boyd JB, Savrin RA, Boles ET Jr (1980) Advances in the management of ga-stroschisis. Ann Surg : 192: 244-248. [Crossref]
- 5. Bruch WS, Langer CJ (2011): Omphalocele and gastroschisis, in: Puri P (ed) Newborn Surgery. Hodder Arnold, London UK 651-660.
- 6. Eurenius K1, Axelsson O (1994) Outcome for fetuses with abdominal wall defects detected by routine second trimester ultrasound. Acta Obstet Gynecol Scand : 73: 25-29. [Crossref]
- 7. Guibourdenche J1, Berrebi D, Vuillard E, de Lagausie P, Aigrain Y, et al. (2006) Biochemical investigations of bowel inflammation in gastroschisis. Pediatr Res : 60: 565-568. [Crossref]
- 8. Langer JC, Longaker MT, Crombleholme TM, Bond SJ, Finkbeiner WE, et al. (1989) "Etiology of intestinal damage in gastroschisis. I: Effects of amniotic fluid exposure and bowel constriction in a fetal lamb model". J pediatr surg : 24: 992-997. [Crossref]
- 9. Tsangaris GT, Kolialexi A, Karamessinis PM, Anagnostopoulos AK, Antsaklis A, et al. (2006) The normal human amniotic fluid supernatant proteome. In Vivo: 20: 479-490. [Crossref]
- 10. Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, et al. (1998) Primary afferent ta-chykinins are required to experience moderate to intense pain. Nature : 392: 390-394. [Crossref]
- 11. Lai Y, Gallo RL (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol : 30: 131-141. [Crossref]
- 12. Xiang Y, Matsui T, Matsuo K, Shimada K, Tohma S, et al. (2007) "Comprehensive investigation of disease-specific short peptides in sera from patients with systemic sclerosis: complement C3f-des-arginine, detected predominantly in systemic sclerosis sera, enhances proliferation of vascu-lar endothelial cells". Arthritis and Rheumat-ism : 56: 2018-2030. [Crossref]
- 13. Takakuwa Y, Kurokawa MS, Ooka S, Sato T, Nagai K, et al. (2011) "AC13, a C-terminal frag-ment of apolipo-protein A-I, is a candidate biomarker for microscopic polyangiitis". Arthritis and Rheumatism : 63: 3613-3624. [Crossref]
- 14. Nagae H, Kitagawa H, Pringle KC, Koike J, Zuccollo J, et al. (2006) "Pressure-limited vesico-amniotic shunt tube for fetal obstructive uropathy". J pediatr surg : 41: 2086-2089. [Crossref]
- 15. Pringle KC, Kitagawa H, Seki Y, Koike J, et al. (2013) "Development of an animal model to study congenital urinary obstruction". Pediatric surgery international : 29:1083-1089. [Crossref]
- 16. Kaneshiro N, Xiang Y, Nagai K, Kurokawa MS, Okamoto K, et al. (2009) Comprehensive anal-ysis of short peptides in sera from patients with IgA nephropathy. Rapid Commun Mass Spectrom: 23: 3720-3728. [Crossref]
- 17. Kitagawa H, Pringle KC, Zuccolo J, Stone P, Nakada K, et al. (1999) The pathogenesis of dys-plastic kidney in a urinary tract obstruction in the female fetal lamb. J Pediatr: Surg 34: 1678-1683. [Crossref]
- 18. Magendzo K, Shirvan A, Cultraro C, Srivastava M, Pollard HB, et al. (1991) Alternative splicing of human synexin mRNA in brain, cardiac, and skeletal muscle alters the unique N-terminal do-main. J Biol Chem: 266: 3228-3232. [Crossref]
- 19. Caohuy H, Pollard HB (2002) Annexin 7: a non-SNARE proteolytic substrate for botulinum toxin type C in secreting chromaffin cells. Ann N Y Acad Sci : 971: 287-290. [Crossref]
- 20. Matsuo K, Arito M, Noyori K, Nakamura H, Kurokawa MS, et al. (2011) Arthritogenicity of an-nexin VII revealed by phosphoproteomics of rheumatoid synoviocytes. Ann Rheum Dis : 70: 1489-1495. [Crossref]
- 21. Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39: 1003-1018. [Crossref]
- 22. Zonneveld R, Martinelli R, Shapiro NI, Kuijpers TW, Plotz FB et al. (2014) "Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults". Critical Care (London, England) : 18: 204.
- 23. Gong F, Pan YH, Huang X, Chen J, Xiao JH, et al. (2013) Interleukin-27 as a potential therapeu-tic target for rheumatoid arthritis: has the time come? Clin Rheumatol : 32: 1425-1428. [Crossref]
- 24. Hashizume C, Moyori A, Kobayashi A, Yamakoshi N, Endo A, et al. (2013) Nucleoporin Nup62 maintains centrosome homeostasis. Cell Cycle : 12: 3804-3816. [Crossref]





