Abstract
Background: In the selection of an appropriate IUD little consideration is placed on adequacy of fit. Properly fitting IUDs will likely lead to less adverse effects or patient discomfort resulting in enhanced continuation of use.
Methods: A multicenter study conducted at 3 centers in 152 nulliparous women, to measure the maximal width of the uterine cavity using 3D ultrasound.
Results: Measurements were performed by experienced sonographists. The mean width of the uterine cavity in the fundus was 21.6 mm (range 6.0 - 40.0 mm). The median value was 22.0 mm and the IQR 18.0 - 24.8 mm, respectively. Eighty-two % of women had a uterine cavity width between 15 mm and 28 mm, 40 % <20 mm and 6.6 % <15 mm, respectively.
Discussion: Uterine cavities in nulliparous women are narrow and rarely wide enough to fit conventional IUDs. Gross discrepancy between the IUD and the uterine cavity leads to side effects (e.g., expulsion, embedment, bleeding, pain) and early discontinuation. Historically, devices too large for the uterine cavity have been routinely inserted which may account for their 5 year continuation rates being only 40 to 50%. Our study suggests that 3D sonography is a precise method to measure the width of the uterine cavity (although 2D may also be suitable) and may result in the selection of a suitable IUD to maximize continuation of use. Measurement of the cavity width is not necessary with a frameless IUD.
Key words
uterine cavity width, 3D ultrasound, nulliparous women, intrauterine device
Introduction
Since their inception little consideration has been placed on the differences in uterine cavity size or its shape in any individual women when inserting an IUD despite the advice of experts who stated that IUD fit is a critical component in their acceptance [1]. Not until recently, and as a consequence of the availability of inexpensive, noninvasive visualization techniques, has interest in the compatibility of any given IUD with a woman’s uterine cavity, or how this compatibility relates to patient comfort and continuation of use, been assessed.
The side effect profile of an IUD and user tolerance is basically determined by its physical characteristics and its geometric relationship to the host uterine cavity [2]. Thus, the tolerability of any device within the uterine cavity remains as the principle determining factor governing the presence or absence of adverse effects. The size and shape of uterine cavities has been compared with the differences in size and shape of our feet. An IUD that is too large for the uterine cavity will be compressed, embedded, or result in perforation. Ultimately partial or complete expulsions can occur. Ill-fitting devices can lead to patient discomfort caused by cramping, pain and abnormal bleeding which may be exacerbated at the time of her menstruation [3]. The uterus is an active muscle capable of producing significant forces in excess of 70 newtons. If the IUD is ill-fitting it may penetrate the uterine wall or cervix [4]. Shipp et al. [5] found that patients with malpositioned and embedded IUDs were more likely to have pain or bleeding than patients with normally positioned devices. Embedment occurs usually very soon after insertion of a too voluminous IUD. In a study conducted in over 400 parous and nulliparous women, more than 50% of women had apparent embedment of framed T-shape devices as assessed by 3D ultrasound examination only 6 weeks after insertion of the IUD. The authors commented that it is unknown if the embedment represents the presence of a penetrating transverse arm or a true secondary perforation [6]. Conversely, women with a transverse diameter of the uterine cavity in the fundus that is greater than the width of the IUD may have an enhanced risk of expulsion or displacement. This was the case for MLCu375 users with transverse uterine widths ≥27 mm and for TCu380A users with cavity widths ≥37 mm [7].
Many IUD trials in young women have produced discouraging results likely as a consequence of using IUDs that did not fit properly. Precisely these young women are most disadvantaged by the occurrence of an unintended pregnancy caused by failure of the IUD [8-13]. Despite the many advantages for using IUDs, the continuation rates of the major IUDs used worldwide (Mirena®, Bayer Healthcare, Germany and TCu380A/Paragard®, Teva Pharmaceuticals, USA) for 5 years of use is only 50% and 40%, respectively [14]. Optimization in IUD selection is clearly required. This study of the width of the uterine cavity in nulliparous women was undertaken to explain the geometric foundation of IUD side effects which may lead to premature discontinuation.
Materials and methods
This non-intervention ultrasound study was conducted by 3 different investigators in Germany and Switzerland. All patients were recruited from a pool of mostly young women either requesting IUD contraception, or who already use an intrauterine contraceptive and are requesting a replacement, or who presented with IUD problems. Transvaginal 3D ultrasound measurements were done at any time during the menstrual cycle. The coronal view of the uterus is particularly well-suited to measure the width of the cavity and to demonstrate the relationship between the entire IUD and the uterine cavity [5]. The uterine cavity width is the distance between the two internal tubal ostia. All investigators used fully optimized and calibrated ultrasonographic equipment.
The study intended to determine the variability in the maximal fundal size in a large group of nulliparous women irrespective of age, body weight or other demographic information.
Data analysis
All 3D coronal images were saved for review. Individual investigators determined the maximal transverse fundal width for their respective patient populations. The findings were collated and all pertinent data included in an Excel spread sheet. Upon study completion the findings were subjected to statistical analysis by an independent statistician, which was performed using R V.3.3.0 (R Foundation for Statistical Computing, Vienna, Austria). Besides descriptive parameters, data were analyzed with the nonparametric Mann-Whitney U test and Kruskal-Wallis test and parametric ANOVA. The significance level was set at a = 0.05 [15].
Results
One hundred and fifty-two (152) nulliparous women participated in this study. The mean age was 25.5 years (SD 5.9 y, range 15-48 y). Figure 1 illustrates the individual patient data. Maximal uterine cavity widths ranged from a low of 6 mm to a maximum of 40 mm.
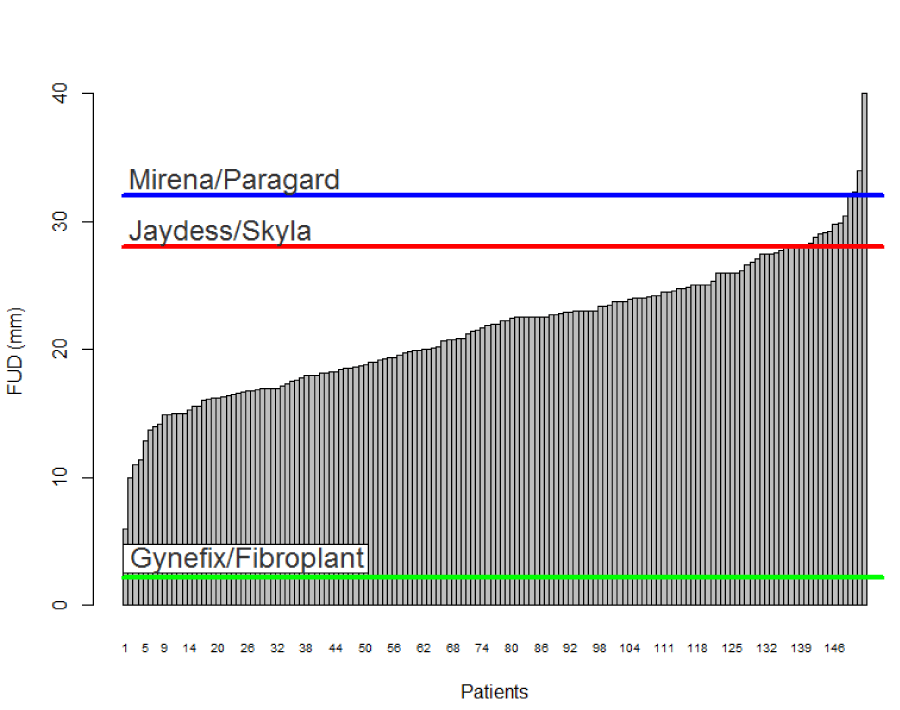
Figure 1.Collated individual maximal fundal widths by 3D sonography in 152 nulliparous women seeking IUD insertion or replacement. For comparison the transverse width for Mirena/Paragard (TCu380A), 32mm, Jaydess/Skyla, 28mm, and the frameless GyneFix (copper)/Fibroplant (LNG), 2.2 mm, are included.
The mean maximal fundal width was found to be 21.6 mm (SD 5.1 mm). Forty % of uterine cavities were less than 20 mm wide; 6.6% less than 15 mm; and 0.7% less than 10 mm, respectively. Only 3 subjects or 2.0% had uterine widths greater than 32 mm. The interquartile range shows that 50% of uterine cavities in this group are between 18.0 mm and 24.8 mm wide, respectively. In our group 82% of patients had a uterine cavity width between 15 mm and 28 mm, respectively.
Figure 2 shows the fundal transverse diameter and range per investigator. Minor, non-significant differences were seen between the investigators (Kruskal-Wallis ANOVA, P = 0.575 [NS]).
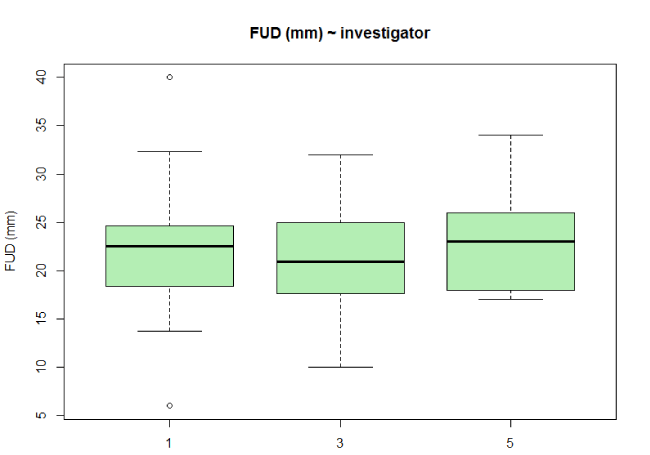
Figure 2. Standard Box plots of patients maximal fundal uterine width by individual investigators.
Table 1 shows the 3D results.
FUD (mm) |
3D |
N |
152 |
Mean |
21.6 |
SD |
5.1 |
Median |
22.0 |
IQR |
18.0 - 24.8 |
Range |
6.0 - 40.0 |
Table 1. Maximal fundal width (FUD) in 152 nulliparous women as measured by 3D office sonography.
Discussion
The use of long-acting reversible contraceptive methods (LARC) are considered of major importance in order to reduce the global “epidemic” of unintended pregnancies, particularly in young women, as they are highly effective and not subject to daily concordance [16]. When viewed in relationship to other contraceptive options, many clinicians and researchers alike view IUDs as the almost near perfect contraceptive system. If true, then why do women using conventional T-shape LNG devices such as Mirena and Levosert/Liletta® (Actavis, USA) or the copper IUD TCu380A/ Paragard fail to use them for their full effective lifespan beyond simply their desire to conceive? Typical discontinuation rates for medical reasons on average for these devices are 10-15% annually [14]. Medical reasons such as cramping, pain, bleeding are the principal reasons that many women discontinue using IUDs. Although still definitively unproven it appears obvious that incompatibility of the IUD itself with the uterine cavity is a significant contributory factor.
Nearly 50 years ago, researchers stressed the importance of an optimal interrelationship between the IUD and the uterine cavity as fewer side effects and greater acceptability would thereby be promoted.1 This study demonstrates the great variation in fundal width of uterine cavities (Figure 3).
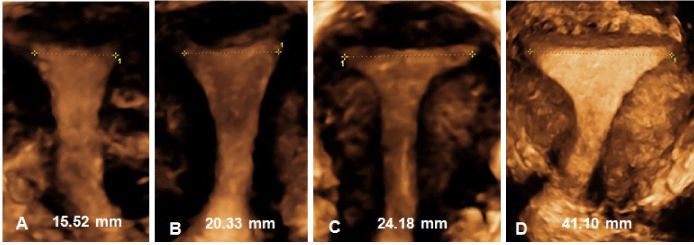
Figure 3. (A-D) 3D examples of measurements of uterine cavities at the fundal level in nulliparous women. The first three pictures (A-C) represent the size of over 70% of women; the picture on the right (D) shows a nulliparous uterine cavity which is exceptionally wide.
Great disparity between the size of the IUD and the uterine cavity will often lead to early removal of the device (Figure 4).
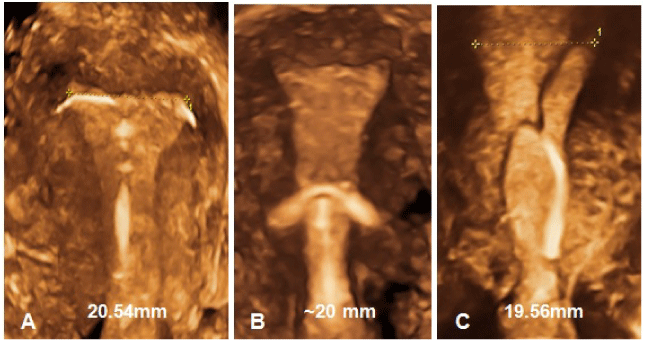
Figure 4. Examples of disparity between the IUD and the uterine cavity leading to complaints and early removal. A) 3D showing transverse arms of T-shape IUD embedded in the uterine cornua; B) 3D of embedded T-shaped IUD (courtesy of Drs. Benacerraf & Shipp); C) 3D of a T-shaped IUD with transverse arms unfolded causing embedment due to lack of space.
Early removal due to cramping pain occurs frequently and more often in nulliparous and adolescent women than in older women. Discontinuation rates after 6-12 months of use of 40 to 50% are not atypical [17]. Early discontinuation places these young women at risk of unintended pregnancy as many among them move to less effective methods or to no protection at all. More importantly, these women will rarely return to intrauterine contraception. They are also spreading bad publicity about the method. Early discontinuation serves to undermine the clear contraceptive advantages of IUDs with a subpopulation of women failing to consider them as viable options. In addition, the wasted expense of the IUD and the burden of insertion fails to provide the patient and third party payers with maximal economic benefit. Providers of IUDs should realize that the only way to obtain comfort during IUD use, and high user continuation, is by using an IUD that is not substantially wider than the width of the uterine cavity.
A growing body of research and clinical evidence is accumulating demonstrating that the uterine cavity for the majority of women is much smaller than many of the IUDs frequently used. A recent 2D ultrasound study conducted in nulliparous women found that about two thirds had a uterine cavity width of less than 24.4 mm. Ultrasound measurement in this group of 165 nulliparous women ranged between 13 and 35 mm [18]. Studies conducted by Kurz in Germany in the 1980s using a measuring instrument inserted in the uterus found a mean fundal transverse diameter in nulliparous women with age between 15 and 39 years of age of between 24 and 25 mm [19]. Our study is in agreement with these previous findings yielding a mean width of 21.6 mm. Surprisingly we found uterine cavities as narrow as 6 mm with 6.6% of the women being below 15 mm. This finding is significant when one realizes that if a device is inserted unknowingly in these women it will be almost 2 cm larger than her cavity itself. Even our mean findings of 21.6 mm demonstrates that over 50% of the women will have maximal uterine widths >1 full cm narrower than the size of Mirena or TCu380A (32 mm). Unfortunately, the optimal IUD size for a specific woman is presently unknown. The muscular nature of the uterus itself may allow for insertion of a larger size device because of its ability to accommodate and is apparently what has been occurring historically in the clinic. Logic suggests that consequences, likely negative, will occur to the patient some of which may result in discontinuation or possibly more drastic medical interventions. With respect to conventional framed T-shaped systems, the precise IUD which will elicit maximal patient comfort while minimizing the risk of expulsion is unknown. In our study we found only 3 women who had maximal fundal widths greater than 32 mm. A critical, but yet unknown balance is required for T-shaped devices capable of long term comfort and retention.
Figure 5 shows uteri of nulliparous women with much different IUD cavity widths, examples of uteri from our daily practices. Most nulliparous women (~80%) in our study had a uterine cavity between 15 mm and 28 mm. What is readily apparent from our study using 3D ultrasound, not only is the size of the uterine cavity variable amongst nulliparous women, so is its shape. Few had uterine shapes comparable to those illustrated in medical textbooks and advertisements.
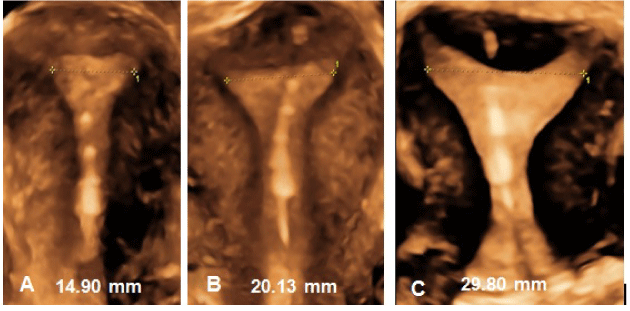
Figure 5. (A-C) Uterine cavities of nulliparous women with different sizes and shapes, varying in cavity width from 15 to 30 mm, which is the width of the uterine cavity in over 80% of women. All contain the frameless IUD.
Conventional T-shaped IUDs are limited by their requirement that they have a transverse arm in order to maintain retention within the uterine cavity. An alternate approach for retention, which eliminates the need entirely for any transverse arms, and can be inserted in any size or shape uterine cavity, is the frameless GyneFix® (Contrel, Ghent, Belgium), marketed as a unique copper device capable of a high degree of effectiveness and high patient acceptance [20]. It utilizes free moving multiple copper cylinders around a nonabsorable suture allowing it to fit different sizes and shapes of the uterus. Its small size, narrow dimensions (2.2 mm) and its flexible nature once inserted allows it to readily adapt to changes in the uterine cavity. In contrast to conventional T-shaped framed IUDs, frameless IUDs maintain a high rate of continuation over the full lifespan of the IUD. Patient continuation rates for frameless devices appear to be substantially greater than that seen for framed systems with continuation rates being reported of over 90% at 5 years [21,22]. Figure 6 shows hysteroscopic views of the frameless GyneFix IUD in a small cavity in comparison with the Mirena IUD inserted in a sufficiently wide uterine cavity. From these pictures, it is clear that the framed Mirena IUD will likely be deformed, expelled or embed if it were inserted in a uterine cavity which is not wide enough. Discrepancy between the size of the IUD and that of the uterine cavity explains the many discontinuations reported in clinical studies in young women [8-13]. In our study several women (0.7%) had maximal fundal widths below 10 mm who had the frameless IUD.
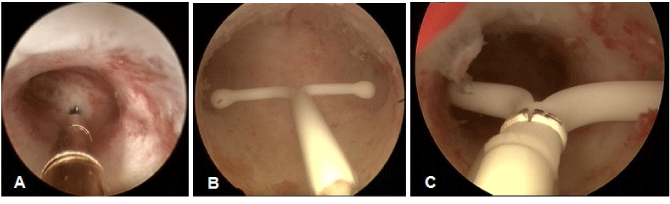
Figure 6. (A) Hysteroscopic view of the frameless copper IUD inserted in a narrow uterus. (B) Hysteroscopic view of the Mirena IUD inserted in a sufficiently large uterine cavity; (C) Hysteroscopic view of Jaydess/Skyla displaced and embedded in the cervix.
The recent introduction of Jaydess/Skyla with a transverse arm of 28 mm is positioned for shorter term use (3 years) in younger women. Although data is still being acquired the patient continuation rates for Jaydess over its 3 year lifespan is ~70 to 75%, a value consistent with the annual discontinuation rate of 10-15% seen with larger devices [23]. Discontinuation rates of ~17% have been reported in one study over a 12 month period in 304 adolescent girls [24]. Even the more recently introduced intrauterine ball device which relies on the use of a memory wire to maintaining shape may provide an option, but recent data suggest that expulsion and request for removal for this device are approaching 50% of participants [25].
The strength of the present study is that 3D measurements were performed by competent sonographers with a great deal of experience in 3D sonography and the use of adequate equipment by all investigators. 3D ultrasound is generally recognized as the gold standard for the evaluation of the uterine cavity. The ability to conduct sonographic measurements in most gynecological practices and clinics affords the clinician an opportunity to assess the suitability of the uterine cavity prior to as well as immediately post insertion and with minimal inconvenience. Our experience also suggests that either 2D or 3D ultrasound are suitable. The boundaries of the uterine cavity can be accurately visualized premenstrual with 2D ultrasound although instillation of gel may be necessary in the first half of the menstrual cycle. Instillation with Instillagel® (Farco Pharma, Köln, Germany) allows mostly good visibility and clear definition of the uterine cavity boundaries (Figure 7).
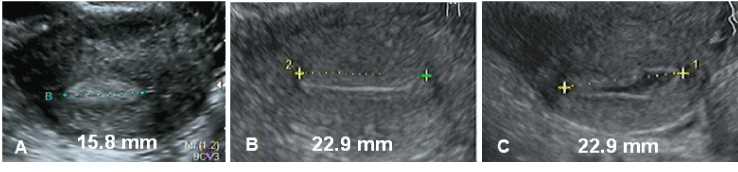
Figure 7. (A) 2D transverse ultrasound with cavity width of 15.8 mm taken in second half of the cycle; (B) 2D transverse ultrasound taken in the first half of the cycle measuring a cavity width of 22.9 mm; (C) ibid. same patient following instillation with gel, showing more clearly the boundaries of the uterine cavity.
3D ultrasonography, however, has added advantages in its ability to fully visualize the entire cavity and IUD simultaneously. It affords the gynecologists the ability to routinely check placement not only at placement but follow-up visits. As utilization of sonography occurs, and physicians become more aware of the importance of uterine cavity with respect to IUD selection, more information on suitable limits with respect to IUD size and shape can be generated.
There are some minimal discrepancies between our measurements of the mean uterine cavity width with those found in previous studies using 2D ultrasound or a measuring instrument (Cavimeter®) [18-19]. However, there is little doubt that the maximal uterine fundal width of most women is much less than first believed. The overall uniformity of our data across independent centers serves to further substantiate the findings.
3D ultrasound and hysteroscopy are the best methods to visualize and evaluate the suitability of IUD in the presence of acquired or congenital anomalies of the uterus. With respect to uterine suitability, uterine anomalies such as uterus arcuatus or a partial septate uterus may reduce the available space in the uterus for conventional IUDs further (Figure 8). These mullerian anomalies are probably more frequent than the incidence of 5% mentioned in the literature [26,27]. It should be noted that bimanual examination is not useful to assess the size of the uterine cavity as there is no relationship between the size of the body of the uterus and the size of the uterine cavity [28].
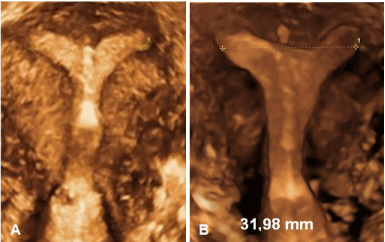
Figure 8. (A) 3D of uterus subseptus with frameless IUD in the midline: the placement of a conventional IUD would be problematic in this case); (B) 3D of uterus arcuatus with intercornual distance of 31.98 mm showing presence of frameless IUD in the midline.
Summary statement
Conventional IUDs are generally too large for uterine cavities of nulliparous women (and many parous women). The use of appropriate intrauterine devices that take into account the geometric relationship of the device to the host uterine cavity will likely result in high rates of continuation due to greater patient comfort leading to fewer unintended pregnancies and induced abortions. At least 2D, preferable 3D equipment, as well as highly trained health care providers, should be available at all sites providing intrauterine contraception as the assessment of a patient’s uterine cavity size is essential in the selection of an appropriate IUD. When selecting a framed IUD, consideration to uterine compatibility should be made. It is evident that measurement of the cavity width is not necessary with the frameless IUD. Routine and repeat ultrasound confirmation of IUD placement, whether 2D or 3D, should be made in an effort to maximize patient comfort.
Conflict of interest
Kilian Nolte, MD, Sohela Jandi, MD, Olivier Julen, MD, Thomas Hasskamp, MD: nil. Dirk Wildemeersch, MD, PhD has conducted research in the field of non-hormonal and hormonal, framed and frameless intrauterine devices, including in nulliparous and adolescent women, since more than 20 years and is the inventor of the GyneFix® IUD. He did not receive any financial compensation of any kind.
Acknowledgement
The authors are grateful to Prof. Dr. G. Van Maele, Department of Medical Informatics and Statistics, University Hospital Ghent, Belgium, for statistical analysis of the data.
References:
2021 Copyright OAT. All rights reserv
- Tejuja S, Malkani PK (1969) Clinical significance of correlation between size of uterine cavity and IUCD: a study by planimeter-hysterogram technique. Am J Obstet Gynecol 105: 620–627. [Crossref]
- Hasson HM (1984) Clinical studies of the Wing Sound II metrology device. In: Zatuchni GI, Goldsmith A, Sciarra JJ, editors. Intrauterine Contraception: Advances and Future Prospects. Philadelphia: Harper and Row, 126–141.
- Goldstuck ND, Wildemeersch D (2014) Role of uterine forces in intrauterine device embedment, perforation, and expulsion. Int J Women’s Health 6: 735–744. [Crossref]
- Wildemeersch D, Hasskamp T, Goldstuck N (2015) Intrauterine devices that do not fit well cause side effects, become embedded, or are expelled and can even perforate the uterine wall. J Minim Invasive Gynecol 22: 309-310. [Crossref]
- Shipp TD1, Bromley B, Benacerraf BR (2010) The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD. J Ultrasound Med 29: 1453-1456. [Crossref]
- Van Schoubroeck D, Van den Bosch T, Ameye L, Veldman J, Hindryckx A, et al. (2013) Pain and bleeding pattern related to levonorgestrel intrauterine system (LNG-IUS) insertion. Eur J Obstet Gynecol Reprod Biol 171: 154-156. [Crossref]
- Liang H1, Li L, Yuan W, Zou Y, Gao ES, et al. (2014) Dimensions of the endometrial cavity and intrauterine device expulsion or removal for displacement: a nested case-control study. BJOG 121: 997-1004. [Crossref]
- Teal SB1, Sheeder J (2012) IUD use in adolescent mothers: retention, failure and reasons for discontinuation. Contraception 85: 270-274. [Crossref]
- Brockmeyer A1, Kishen M, Webb A (2008) Experience of IUD/IUS insertions and clinical performance in nulliparous women--a pilot study. Eur J Contracept Reprod Health Care 13: 248-254. [Crossref]
- Garbers S1, Haines-Stephan J, Lipton Y, Meserve A, Spieler L, et al. (2013) Continuation of copper-containing intrauterine devices at 6 months. Contraception 87: 101-106. [Crossref]
- Berenson AB1, Tan A, Hirth JM, Wilkinson GS (2013) Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol 121: 951-958. [Crossref]
- Rasheed SM, Abdelmonem AM (2011) Complications among adolescents using copper intrauterine contraceptive devices. Int J Gynaecol Obstet 115: 269–272. [Crossref]
- Aoun J, Dines VA, Stovall DW, Mete M, Nelson CB, et al. (2014) Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 123: 585-592. [Crossref]
- Diedrich JT, Madden T, Zhao Q, Peipert JF (2015) Long-term utilization and continuation of intrauterine devices. Am J Obstet Gynecol 213: 822. [Crossref]
- Dean CB, Nielsen JD (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 13: 497-512. [Crossref]
- Blumenthal PD, Voedisch A, Gemzell-Danielsson K (2011) Strategies to prevent unintended pregnancy: increasing use of long-acting reversible contraception. Hum Reprod Update 17: 121-137. [Crossref]
- Wildemeersch D, Pett A, Jandi S, Hasskamp T, Rowe P, et al. (2013) Precision intrauterine contraception may significantly increase continuation of use: a review of long-term clinical experience with frameless copper-releasing intrauterine contraception devices. Int J Women’s Health 5: 215–225. [Crossref]
- Kaislasuo J, Heikinheimo O, Lähteenmäki P, Suhonen S (2014) Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstet Gynecol 124: 345-353. [Crossref]
- Kurz KH (1984) Cavimeter uterine measurements and IUD clinical correlation. In: Zatuchni GI, Goldsmith A, Sciarra JJ, editors. Intrauterine Contraception: Advances and Future Prospects. Philadelphia: Harper and Row, 142–162.
- Wildemeersch D, Pett A, Jandi S, Hasskamp T, Rowe P, et al. (2013) Precision intrauterine contraception may significantly increase continuation of use: a review of long-term clinical experience with frameless copper-releasing intrauterine contraception devices. Int J Women’s Health 5:215–225. [Crossref]
- Cao X1, Zhang W, Zhao X, Lin N, Wang L, et al. (2004) Three-year efficacy and acceptability of the GyneFix 200 intrauterine system. Contraception 69: 207-211. [Crossref]
- Wildemeersch D, Jandi S, Pett A, Nolte K, Hasskamp T (2014) Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: results of a multicenter study. Int J Womens Health 6: 727–734. [Crossref]
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, et al. (2013) Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol 122: 1205–1213. [Crossref]
- Gemzell-Danielsson K, Buhling KJ, Dermout SM, Lukkari-Lax E, Montegriffo E, et al. (2016) A Phase III, single-arm study of LNG-IUS 8, a low dose levonorgestrel intrauterine contraceptive system (total content 13.5 mg) in postmenarcheal adolescents. Contraception 93: 507–512. [Crossref]
- Wiebe E, Trussell J (2016) Discontinuation rates and acceptability during 1 year of using the intrauterine ball (the SCu380A). Contraception 93: 364-366. [Crossref]
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, et al. (2011) The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update 17: 761-771. [Crossref]
- Jurkovic D, Gruboeck K, Tailor A, Nicolaides KH (1997) Ultrasound screening for congenital uterine anomalies. Br J Obstet Gynaecol 104: 1320-1321. [Crossref]
- Benacerraf BR, Shipp TD, Lyons JG, Bromley B (2010) Width of the normal uterine cavity in premenopausal women and effect of parity. Obstet Gynecol 116: 305-310. [Crossref]








