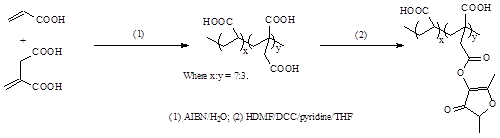A glass-ionomer cement containing a natural fruit component - 4-hydroxy-2,5-dimethyl-3(2H)-furanone has been developed. Compressive strength (CS) and S. mutans viability were used to evaluate the mechanical strength and antibacterial activity of the formed cement. The formulated antibacterial cement showed a significant antibacterial activity, accompanying with an initial CS reduction. Increasing the compound loading significantly decreased the S. mutans viability from 5 to 81% and also reduced the initial CS of the formed cements from 4 to 58%. The cement loaded with 7% antibacterial polymer showed 168 MPa, 7.8 GPa, 243 MPa, 46 MPa, and 57 MPa in yield strength, modulus, CS, diametral tensile strength and flexural strength, respectively, as compared to 141, 6.9, 236, 42 and 53 for Fuji II LC. The cement also showed a similar antibacterial activity to S. mutans, lactobacillus, S. aureus and S. epidermidis. The human saliva did not affect the antibacterial activity of the cement. The thirty-day aging study indicates that the experimental cement may have a long-lasting antibacterial function.
4-hydroxy-2,5-dimethyl-3(2H)-furanone derivative, antibacterial polymer, S. mutans viability, glass-ionomer cement, compressive strength
Abbreviations
MO: Oral mucositis; OI: odontogenic infections; HCPA: Hospital de Clínicas de Porto Alegre;
ALL: acute lymphocytic leukemia; POS-HCPA: Pediatric Oncology Service - Hospital de Clínicas de Porto Alegre; AOH: appropriate oral health; IHO: inappropriate oral health; WHO: World Health Organization; HR: hazard ratio; OHH: Oral hygiene habits ; DMFT index: decayed, missing, and filled teeth index; VPS: visible plaque score; GBI: gingival bleeding index; LILT: low-intensity laser therapy
Secondary caries is found to be one of the main reasons to the restoration failure of dental restoratives including resin composites and glass-ionomer cements [1-4]. Secondary caries often occurs at the interface between the restoration and the cavity preparation. One of the main reasons to cause secondary caries is demineralization of tooth structure due to invasion of plaque bacteria (acid-producing bacteria) such as Streptococcus mutans (S. mutans) and lactobacilli in the presence of fermentable carbohydrates [4]. These bacteria produce lactic acid which can dissolve hydroxyapatide and thus cause secondary caries [4]. Numerous efforts have been made on improving antibacterial activities of dental restoratives. One strategy was to incorporate antibacterial agents such as antibiotics, zinc ions, silver ions, iodine and chlorhexidine [5-9] to restoratives. Bacteria are killed or inhibited through release or slow-release of these agents. However, release or slow-release can lead or has led to a reduction of mechanical properties of the restoratives over time, short-term effectiveness, and possible toxicity to surrounding tissues if the dose or release is not properly controlled [5-9]. The other strategy was to attach quaternary ammonium salt (QAS) or phosphonium salt groups onto materials or restoratives [10-14]. These materials are found to have broad antibacterial spectrum [12-14] and be capable of reducing the number of bacteria that are resistant to other types of cationic antibacterials [15]. The QAS-containing materials have been successfully applied for dental restoratives including using methacryloyloxydodecyl pyridinium bromide for resin composites [12], methacryloxylethyl cetyl ammonium chloride for antibacterial bonding agents [16,17], quaternary ammonium polyethylenimine nanoparticles [18] and other QAS-containing derivatives [19-23] for resin composites, polyQAS for glass-ionomer cements (GICs) [24]. All these studies found that the QAS-containing materials exhibited significant antibacterial activities. However, it has also been reported that human saliva can significantly decrease the antibacterial activity of the QAS-containing restoratives, probably due to electrostatic interactions between QAS and proteins in saliva [25-26]. Recently furanone derivatives have been found to have strong antitumor [27-28] and antibacterial functions [29]. These compounds all contain a furanone structure (classified as lactone or butenolide) and showed a similarity to natural manoalide, which has an interesting anti-inflammatory activity [30]. The similar compounds were also found to show an inhibitory effect on bacterial quorum-sensing [31], probably due to the structural similarity to autoinducers in bacteria. The exact antibacterial mechanism is still unclear and under investigation. 4-Hydroxy-2,5-dimethyl-3(2H)-furanone or HDMF, extracting from many fruits, was also found to show significant antimicrobial functions to both bacteria and fungi [32] without hemolytic activity to human. In this study, we would like to explore this natural compound in antibacterial application for dental restoratives. The objective of this study was to link HDMF to polyacid, use it to formulate the light-curable glass-ionomer cements, and study the effect of this new antibacterial compound on compressive strength and antibacterial activity of the formed cements. DTS and FS were also determined.
Materials
Acrylic acid (AA), itaconic acid (IA), 2,2’-azobisisobutyronitrile (AIBN), 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF), N,N'-dicyclohexylcarbo diimide (DCC), pyridine, dl-camphoroquinone (CQ), 2-(dimethylamino)ethyl methacrylate (DMAEMA), tetrahydrofuran (THF) and diethyl ether were used as received from Fisher Scientific (Waltham, MA) without further purifications. Commercial glass-ionomer cement Fuji II LC and its corresponding glass powders were used as received from GC America Inc (Alsip, IL).
Synthesis and characterization
Synthesis of antibacterial poly(AA-co-IA) copolymer with pendent HDMF: Poly(AA-co-IA) with a molar ratio of 7:3 was synthesized following our published protocol [33]. The purified polymer was then used to react with HDMF to form the polymer containing HDMF. Briefly, to a solution containing poly(AA-co-IA), pyridine (1% by weight) and DCC (1.1 equivalent to HDMF) in THF, HDMF in THF was added. The reaction was run at room temperature overnight. After the precipitates dicyclohexylurea (DCU) were filtered, the polymer solution was purified by precipitation from diethyl ether, followed by drying in a vacuum oven. The synthesis scheme for poly(AA-co-IA) with pendent HDMF or PAIH is shown in Figure 1.
Figure 1. Schematic diagrams for synthesis of poly(AA-co-IA) with pendent HDMF.
Synthesis of the matrix polymer - star-shaped polymer with pendent methacrylate groups: The light curable star-shaped poly(AA-co-IA) with pendent methacrylate groups was synthesized as described previously [34]. The synthesis involves three steps: (1) synthesis of a star-shaped chain-transfer agent (CTA); (2) synthesis of the star-shaped poly(AA-co-IA) copolymer via free-radical polymerization using the CTA; and (3) grafting of pendent methacrylate groups onto the star-shaped poly(AA-co-IA).
Characterization: HDMF, poly(AA-co-IA) and PAIH were characterized by Fourier transform-infrared (FT-IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. The formed polymers were also characterized by gel permeation chromatography (GPC). The proton NMR (1HNMR) spectra were obtained on a 500 MHz Bruker NMR spectrometer (Bruker Avance II, Bruker BioSpin Corporation, Billerica, MA) using deuterated dimethyl sulfoxide as solvents and FT-IR spectra were obtained on a FT-IR spectrometer (Mattson Research Series FT/IR 1000, Madison, WI). Molecular weights of the formed polymers were determined following the published protocol [35], on a Waters GPC unit (Model 410 differential refractometer, Waters Inc., Milford, MA) with THF as a solvent, using standard GPC techniques and polystyrene standards.
Evaluation
Cement sample preparation for strength and antibacterial tests: The experimental cements were formulated with a two-component system (liquid and powder) [36]. The liquid was formulated with the matrix polymer - light-curable star-shaped poly(AA-co-IA), water, 0.9% CQ (photo-initiator, by weight) and 1.8% DMAEMA (activator). The polymer/water (P/W) ratio = 65:35 (by weight). Fuji II LC glass powder was either used alone or mixed with the synthesized antibacterial polymer PAIH to formulate the cements, where the PAIH loading ratio = 1, 3, 5, 7, 10, 15%, 20%, 25% or 30% (by weight) of the glass. Glass powder powder/polymer liquid (P/L) ratio = 2.7 (by weight).
Specimens were fabricated at room temperature according to the published protocol [36]. Briefly, the cylindrical specimens were prepared in glass tubing with dimensions of 4 mm in diameter by 8 mm in length for compressive strength (CS), 4 mm in diameter by 2 mm in length for diametral tensile strength (DTS), and 4 mm in diameter by 2 mm in depth for antibacterial tests. The rectangular specimens were prepared in a split Teflon mold with dimensions of 3 mm in width by 3 mm in thickness by 25 mm in length for flexural strength (FS) test. All the specimens were exposed to blue light (LED, 30W, EXAKT 520 Blue Light Polymerization Unit, EXAKT Technologies, Inc., Oklahoma City, OK) for 2 min, followed by conditioning in 100% humidity at room temperature for 15 min, removing from the mold and conditioning in distilled water at 37°C for 24 h prior to testing, unless specified.
Strength measurements
CS, DTS and FS tests were performed on a screw-driven mechanical tester (QTest QT/10, MTS Systems Corp., Eden Prairie, MN), with a crosshead speed of 1 mm/min. The FS test was performed in three-point bending with a span of 20 mm between supports. Six specimens were tested to obtain a mean value for each material or formulation in each test. CS was calculated using an equation of CS = P/pr2, where P = the load at fracture and r = the radius of the cylinder. DTS was determined from the relationship DTS = 2P/pdt, where P = the load at fracture, d = the diameter of the cylinder, and t = the thickness of the cylinder. FS was obtained using the expression FS = 3Pl/2bd2, where P = the load at fracture, l = the distance between the two supports, b = the breadth of the specimen, and d = the depth of the specimen. Compressive yield strength (YS) and modulus (M), were obtained from the stress-strain curves of the CS tests.
MIC test for the evaluated antibacterial monomers
The minimal inhibitory concentration (MIC) of both natural HDMF and PAIH was determined following the published protocol with a slight modification [24]. Briefly, colonies of S. mutans (UA159) were suspended in 5 ml of Tryptic soy Broth (TSB) prior to MIC testing. Two-fold serial dilutions of the synthesized monomer were prepared in TSB, followed by placing in 96-well flat-bottom microtiter plates with a volume of 250 μl per well. The microtiter plate was then inoculated with S. mutans suspension (cell concentration = 5 × 105 CFU/ml) and incubated at 37°C for 48 h prior to MIC testing. The absorbance was measured at 595 nm via a microplate reader (SpectraMax 190, Molecular Devices, CA) to assess the cell growth. Chlorhexidine and dimethylsulfoxide were used as positive and negative controls, respectively. Triple replica was used to obtain a mean value for each material. Other bacteria including lactobacillus, Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis) were also used for MIC evaluations.
Antibacterial test
The antibacterial test was conducted following the published procedures [24]. S. mutans was mainly used to evaluate the antibacterial activity of the studied cements throughout the study. Other bacteria including lactobacillus, S. aureus and S. epidermidis were also used to evaluate a broad antibacterial activity of the studied cements. Briefly, colonies of S. mutans (UA159) were suspended in 5 ml of Tryptic soy Broth (TSB), supplemented with 1% sucrose, to make a suspension with 108 CFU/ml of S. mutans, after 24 h incubation. Each cement specimen was dipped in 70% ethanol for 10 sec, followed by drying in the air for another 10-20 sec and placing in a vial containing 5 ml TSB supplemented with 1% sucrose. To the specimen-containing TSB, 100 μl of the above incubated S. mutans suspension was added. After incubating at 37oC for 48 h under anaerobic condition with 5% CO2, the specimen-containing suspension was sonicated for 20 sec to remove the adhered bacteria off the specimen. 1 ml of the suspension was then used to mix with 3 μl of a two-color dye, which was formed by thoroughly mixing equal volumes of the red and the green dyes (LIVE/DEAD BacLight bacterial viability kit L7007, Molecular Probes, Inc., Eugene, OR, USA) in a microfuge tube for 1 min. The formed mixture was vortexed for 10 sec, sonicated for 10 sec, vortexed for another 10 sec, and kept in dark for about 15 min, prior to analysis. Then 20 μl of the stained bacterial suspension was analyzed using a fluorescent microscope (Nikon Microphot-FXA, Melville, NY, USA). Triple replica was used to obtain a mean value for each material.
Saliva effect test
Human saliva, obtained from a healthy volunteer, was centrifuged for 15 min at 12,000g to remove debris [25]. After the supernatant was filtered with a 0.45 μm sterile filter, the filtrate was stored in a -20oC freezer prior to use. The sterilized cement specimen (see Antibacterial test) was incubated in a small tube containing 1 ml of saliva at 37oC for 2 h, followed by placing in 5 ml TSB supplemented with 1% sucrose. The rest of the procedures for antibacterial test were the same as described before.
Aging of the specimens
The specimens for both CS and antibacterial activity aging tests were conditioned in distilled water at 37oC for 1, 3, 7 14 and 30 d, followed by direct testing for CS (see CS testing for details) and incubating with S. mutans for 48 h for antibacterial testing (see antibacterial testing for details).
Statistical analysis
One-way analysis of variance (ANOVA) with the post hoc Tukey-Kramer multiple-range test was used to determine significant differences of mechanical strength and antibacterial tests among the materials or formulations in each group. A level of α = 0.05 was used for statistical significance.
Characterization
Figure 2A shows the FT-IR spectra for poly(AA-co-IA), HDMF and PAIH. The characteristic peaks (cm-1) were: (a) HDMF, 3251, 1308, 1151 and 759 (-OH), 2970, 2870, 1451, 1370 and 1002 (-CH and -CH3), 1698 (carbonyl on –C=C-CO-), 1623, 1197 and 1042 (C=C on -C=C-O-C-), 1094 and 929 (ether on -C-O-C-); (b) p(AA-co-IA), 2963 (2385-3687) (OH on –COOH), 2970, 2661, 1450, 1184 and 797 (-CH and CH3), 1719 (carbonyl on –COOH); (c) PAIH, 2937 (2385-3687) (OH on –COOH), 2661, 1450 and 1368 (-CH and -CH3), 1729, 1509 and 1403 (carbonyl on both acrylate and –C=C-CO-), 1604 and 1196 and 1021 (C=C on -C=C-O-C-), 1262 and 939 (ether on -C-O-C-). Disappearance of the broad peak (cm-1) at 3251 for hydroxyl group on HDMF, formation of a broader and wider peak between 3500 and 2750 for COOH, formation of a wider and stronger peak at 1729 for carbonyl groups on both poly(AA-co-IA) and HDMF, and appearance of a new peak at 1604 for C=C group on HDMF confirmed successful attachment of HDMF onto poly(AA-co-IA). Figure 2B shows the 1HNMR spectra for HDMF, poly(AA-co-IA) and PAIH. The chemical shifts (ppm) were: (a) HDMF, 8.25 (-OH), 4.52 (-CH), 2.12 (-CH3) and 1.28 (-CH3); (b) 12.15 (-COOH) and others; (c) PAIH, 12.35 (-COOH), 4.25 (-CH), 2.15 (-CH3), 1.35 (-CH3) and others. Disappearance of the chemical shift at 8.25 (-OH) and formation of all the new chemical shifts on PAIH confirmed the successful attachment of HDMF onto poly(AA-co-IA).
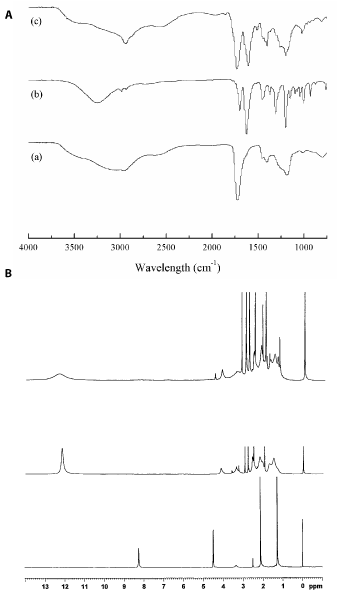
Figure 2. FT-IR and 1HNMR spectra for HDMF, poly(AA-co-IA) and PAIH: (a) poly(AA-co-IA), (b) HDMF, and (c) PAIH.
Evaluation
Table 1 shows the MIC of HDMF, PAIH and CHX to S. mutans, lactobacillus, S. aureus and S. epidermidis. The MIC values ranged from 19 to 39 µg/ml for HDMF, 39 to 78 for PAIH, and 4.9 to 9.8 for chlorhexidine.
Table 1. MIC values of the materials used in the study
Compounds1 |
S. mutans |
lactobacillus |
S. aureus |
S. epidermidis |
HDMF |
19 |
39 |
19 |
19 |
PAIH |
39 |
78 |
39 |
39 |
Chlorhexidine |
4.9 |
9.8 |
4.9 |
4.9 |
1HDMF and PAIH are the abbreviations of antibacterial materials, which can be found under Materials and Methods. MIC values (μg/ml) were measured as described in the text.
Figure 3 shows the effect of PAIH content on CS of the cements. The mean CS was decreased with increasing PAIH content, where there were no statistically significant differences between 0% and 1%, 1% and 3%, 3% and 5%, and 5% and 7% (p > 0.05). The PAIH addition significantly decreased CS with a reduction of 4 to 58%.
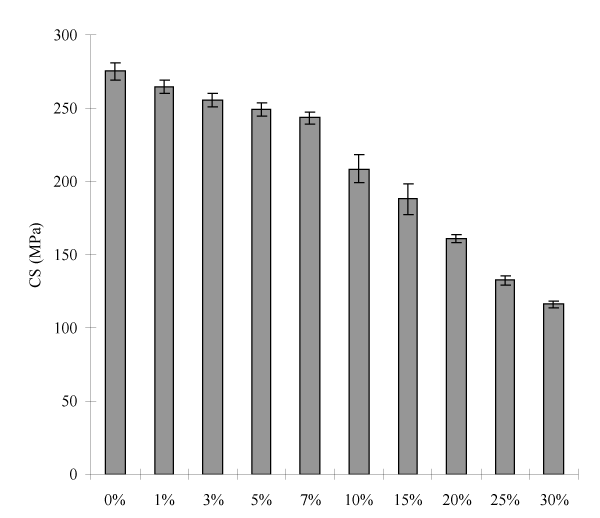
Figure 3. Effect of PAIH content on CS of the experimental cements: PAIH = poly(AA-co-IA) with pendent HDMF; MWs of PAIH and of the star-shaped poly(AA-co-IA) = 22,860 and 31,480 Daltons, respectively; Filler = Fuji II LC or Fuji II LC + PAIH; Grafting ratio = 50%; P/L ratio = 2.7; P/W ratio = 70:30. Specimens were conditioned in distilled water at 37°C for 24 h prior to testing.
Figure 4 shows the effect of PAIH content on the S. mutans viability of the cements. The mean S. mutans viability was decreased with increasing PAIH content, where there were no statistically significant differences between 0% and 1%, 1% and 3%, 3% and 5%, and 25% and 30% (p > 0.05). The PAIH addition significantly decreased the S. mutans viability with a reduction of 5 to 81%.
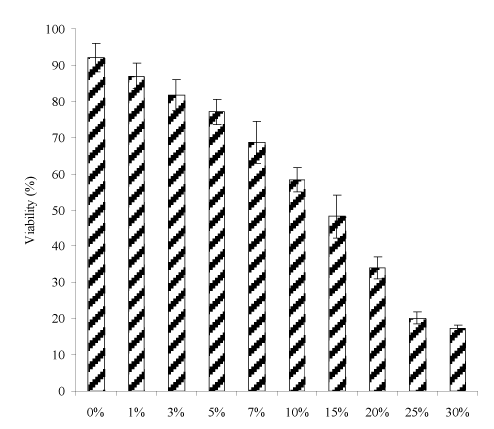
Figure 4. Effect of PAIH content on S. mutans viability of the experimental cements: The formulations were the same as those described in Figure 3. Specimens were conditioned in distilled water at 37°C for 24 h, followed by incubating with S. mutans before antibacterial testing.
Table 2 shows the effects of PAIH on the viability of four bacteria including S. mutans, lactobacillus, S. aureus and S. epidermidis and human saliva on antibacterial cements. The PAIH at 7% in cements reduced the viability of all four bacteria in the range of 46.3 to 83.8. Table 2 also shows that human saliva nearly exerted no effect on the S. mutans viability after culturing with the antibacterial cements. No statistically significant differences in the S. mutans viability were found between the cements with and without human saliva treatment.
Table 2. Effects of PAIH on different bacteria and human saliva on antibacterial cements1
S. mutans |
lactobacillus |
S. aureus |
S. epidermidis |
Cements without saliva treatment |
68.7 (5.7)a,2 |
46.3 (0.8)b |
83.8 (1.2)c |
77.9 (3.9)c,d |
Cements with saliva treatment |
63.1 (2.8)a |
46.9 (2.2)b |
84.3 (1.6)c |
75.1 (4.5)d |
1The formulations were the same as those described in Figure 4, except for PAIH = 7%. 2Entries are mean values with standard deviations in parentheses and the mean values with the same superscript letter were not significantly different (p > 0.05). Specimens were conditioned in distilled water at 37°C for 24 h, followed by incubating with bacteria before antibacterial testing. For saliva treatment, specimens were soaked in human saliva at 37°C for 2 h, followed by incubating with S. mutans before antibacterial testing.
Figure 5 shows the effect of the cement with 7% PAIH aging on CS. The CS value (MPa) was increased from 213 (1 h) to 243 (1 d), 259 (3 d), 264 (7 d), 270 (14 d), and 288 (30 d).
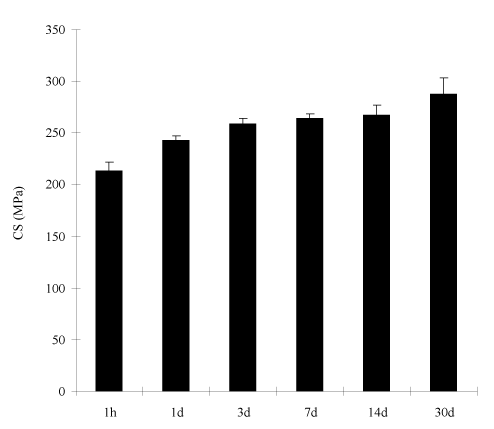
Figure 5. Effect of aging on CS of the experimental cements: The formulations were the same as those described in Figure 3, except for PAIH content = 7%. Specimens were conditioned in distilled water at 37°C for 1 h, 1 d, 3 d, 7 d, 14 d and 30 d prior to testing.
Figure 6 shows the effect of the PAIH cement aging on the S. mutans viability. After 30-day aging in water, no statistically significant changes were found in the S. mutans viability for the cement with 7% PAIH addition.
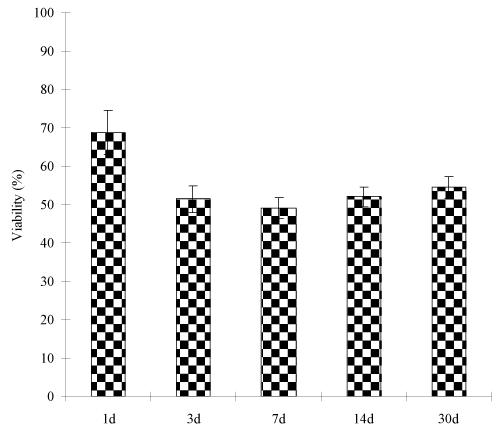
Figure 6. Effect of aging on S. mutans viability of the experimental cements: The formulations were the same as those described in Figure 6. Specimens were conditioned in distilled water at 37°C for 1, 3, 7, 14 and 30 d, following by incubating with S. mutans for 48 h prior to antibacterial testing.
Table 3 shows the property comparison among the cements with 0 and 7% of PAIH addition and Fuji II LC. As compared to the cement with 0% PAIH, the cement with 7% PAIH showed a decrease in all the measured strengths. The decreases of 18%, 9%, 11%, 10% and 20% were observed, respectively, in yield strength (YS), modulus (M), CS, diametral tensile strength (DTS) and flexural strength (FS), among which YS and FS showed more reduction. A significant decrease with a 25% reduction was observed in the S. mutans viability. On the other hand, the experimental antibacterial cement showed higher mechanical strength values than commercial GIC Fuji II LC, with 19%, 12%, 3%, 8% and 7% increase in YS, CM, CS, DTS and FS, and a lower S. mutans viability (24% lower). Fuji II LC showed the similar S. mutans viability to the cement with 0% PAIH.
Table 3. Comparison of properties of the experimental cements with and without PAIH1
Polymer (%) |
YS2 [MPa] |
M3 [GPa] |
CS [MPa] |
DTS4 [MPa] |
FS5 [MPa] |
Viability (%) |
0 |
205.7 (3.6) |
8.52 (0.15) |
275.3 (5.9) |
51.2 (3.5)b |
71.4 (4.6) |
92.1 (4.1)e |
7 |
168.1 (6.6) |
7.75 (0.18) |
243.4 (4.2)a,7 |
46.2 (1.4)b,c |
57.1 (2.8)d |
68.7 (5.7) |
Fuji II LC6 |
141.2 (1.9) |
6.89 (0.38) |
236.2 (3.4)a |
42.8 (0.9)c |
53.3 (2.1)d |
90.9 (0.9)e |
1The formulation for antibacterial cement was the same as those described in Fig. 4; 2YS = CS at yield; 3M = compressive modulus; 4DTS = diametral tensile strength; 5FS = flexural strength; 6Fuji II LC = commercial GIC; 7Entries are mean values with standard deviations in parentheses and the mean values with the same superscript letter were not significantly different (p>0.05). Specimens were conditioned in distilled water at 37°C for 24 h, followed by direct testing for all the strengths and incubating with S. mutans for 48 h for antibacterial testing.
In preventive restorative dentistry, secondary caries is a critical issue and prevention of secondary caries plays a key role in long-lasting restorations [1-4]. There are basically two strategies in fighting bacteria-related secondary caries: one was to release or slow-release antibacterial agents such as antibiotics, zinc ions, silver ions, iodine and chlorhexidine [5-9] and the other to attach QAS onto restoratives [11-24]. However, release or slow-release can lead or has led to a reduction of mechanical properties of the restoratives over time, short-term effectiveness, and possible toxicity to surrounding tissues if the dose or release is not properly controlled [5-9]. The QAS-containing materials have attracted a special attention due to their killing bacteria by touch or simple contact [11-24]. The QAS-containing materials not only inhibit Gram-positive but also kill Gram-negative bacteria [10-19]. These materials were found to be capable of killing bacteria that are resistant to other types of cationic antibacterial agents [15]. They also show a broad antibacterial spectrum [11-20]. However, it was also reported that human saliva can significantly decrease the antibacterial activity of the QAS-containing restoratives, probably due to electrostatic interactions between QAS and proteins in saliva [25-26]. Recently furanone-containing materials were reported to show a broad range of biological and physiological properties including antitumor, antibiotic, haemorrhagic and insecticidal activity [27-28], although the biological mechanism of these derivatives is still under investigation. Natural furanone compound, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, extracting from fruits such as strawberry, was also found to show significant antimicrobial functions to both bacteria and fungi [32]. In current study, we incorporated this compound to GIC by covalent attachment. The following discussion presents how HDMF was incorporated into our experimental GICs and its effect on mechanical and antibacterial properties of the formed cements.
The results shown in Table 1 indicate that both natural HDMF and the synthesized polymer PAIH are potent antibacterial agents, even though they are not as potent as chloehexidine. The four bacteria strains tested belong to Gram-positive bacteria, among which S. mutans and lactobacillus are common oral bacteria which are responsible for oral cavity formation. It has been reported that natural HDMF not only kills Gram-positive but also destroys Gram-negative bacteria and even fungi [32].
Figures 3 and 4 show the effects of PAIH content on CS and S. mutans viability of the cements, respectively. Obviously with PAIH addition the cement showed a decrease in CS and S. mutans viability. However, their decreasing trends are a little different. The cements lost 4 to 58% of its original CS value (275 MPa) with 1 to 30% PAIH addition (Figure 3), among which the cements with 1 to 7% PAIH showed a 4 to 11% loss in CS but the values (264.5 to 243.4) were still above 236 MPa which was shown by Fuji II LC (Table 5). The loss of CS can be attributed to the incorporated PAIH because hydrophobic PAIH did not contribute any strength enhancement to the cements. Regarding the S. mutans viability (Figure 4), PAIH significantly increased the antibacterial activity of the cement. With 1 to 30% PAIH addition, the S. mutans viability was reduced from 5 to 81%, among which the cements with 1 to 7% PAIH showed a 5 to 25% reduction. By examining the results on CS and viability values among 3%, 5% and 7% PAIH addition, the CS changes of these cements were only 2-4% but their effects on S. mutans viability were 6-16% in difference. In other words, if we added 7% PAIH into the cement, we would have an antibacterial cement not only with CS (243.4) better than Fuji II LC (236.2) but also having an enhanced antibacterial activity (68.7% vs. 90.9%). Therefore, the cement with 7% PAIH addition was chosen to further evaluate the other properties.
Table 2 shows the effects of PAIH on different bacteria and human saliva on bacterial viability after culturing with the PAIH cements. It is known that lactobacillus is another main oral cavity-producing bacterium although it is not as popular as S. mutans. S. aureus and S. epidermidis are two major bacteria that often cause skin and implant infections. To examine the antibacterial activity of PAIH on these bacteria, we compared the viability of all the four bacteria after incubating with the cements. With 7% PAIH addition, lactobacillus showed the lowest viability (46.3%), followed by S. mutans, S. epidermidis and S. aureus. The results are somehow different from those shown on the MIC test. Regarding human saliva evaluation, no statistically significant differences in bacterial viability were found between the cements with and without human saliva treatment. It has been noticed that saliva can significantly reduce the antibacterial activity of the QAS-containing materials based on the mechanism of contact inhibition [25,26]. The reduction was attributed to the interaction between positive charges on QAS and amphiphilic protein macromolecules in saliva, thus leading to formation of a protein coating which covers the antibacterial sites on QAS [25,26]. Unlike QAS, PAIH does not carry any charges. That may be why the PAIH-modified cements did not show any reduction in antibacterial activity after treating with saliva.
It is known that GICs increase their strengths with time due to constant salt-bridge formations [37]. To confirm if the PAIH-modified GIC still follows the pattern that most GICs exhibit, we examined both CS and antibacterial activity of the cements after aging in water for 1 hour, 1 day, 3 days, 7 days, 14 days and 30 days. The result in Figure 5 shows that the cements with 7% PAIH showed 35% increase in CS after 30-day aging in water, compared to 1-h aging. It also shows that the cement had 14% and 24% increase for 1-day and 7-day aging, compared to 1-h aging. The result is consistent with those reported earlier [38] and elsewhere [39]. Meanwhile no statistically significant changes in the S. mutans viability were found during the 30-day aging (Figure 6). The reason can be attributed to the fact that PAIH is a copolymer of acrylic acid and DMFA. It is known that the carboxylic acid group plays a key role in GIC setting and salt-bridge formation. PAIH not only provides antibacterial function but also supplies carboxyl groups for salt-bridge formation. When PAIH was mixed into the cement, the carboxyl groups of PAIH helped the polymer to firmly attach to the glass fillers by forming salt-bridges. The above results also imply that PAIH did not leach out of the cement; otherwise both CS and antibacterial activity would show a decreasing trend.
Finally we compared YS, M, CS, DTS, FS and the S. mutans viability of the experimental cements with 0 and 7% PAIH and Fuji II LC. As shown in Table 3, the PAIH-modified cement was 18% in YS, 9% in modulus, 11% in CS, 9.8% in DTS and 20% in FS lower than the cement without PAIH addition. On the other hand, the PAIH-modified cement was much higher (25% higher) in antibacterial activity than the cement without PAIH addition. As compared to commercial GIC Fuji II LC, the cement with 7% PAIH showed 19% in CS, 12% in modulus, 3% in CS, 8% in DTS and 7% in FS higher than Fuji II LC but 24% lower in S. mutans viability values.
We have developed an antibacterial glass-ionomer cement containing a natural fruit component 4-hydroxy-2,5-dimethyl-3(2H)-furanone. The modified cement showed a significant antibacterial activity, accompanying with an initial CS reduction. Increasing loading of 4-hydroxy-2,5-dimethyl-3(2H)-furanone significantly enhanced antibacterial activity but reduced the initial CS of the formed cement. The experimental cement showed a similar antibacterial activity to S. mutans, lactobacillus, S. aureus and S. epidermidis. The human saliva did not affect the antibacterial activity of the cement. The 30-day aging study indicates that the experimental antibacterial cement may have a long-lasting antibacterial function.
This work was partially sponsored by NIH grant DE020614 and Dr. Gregory at Indiana University School of Dentistry was acknowledged for providing laboratory for the antibacterial tests.
- Mjör IA, Dahl JE, Moorhead JE (2002) Placement and replacement of restorations in primary teeth. Acta Odontol Scand 60: 25-28. [Crossref]
- Forss H, Widström E (2004) Reasons for restorative therapy and the longevity of restorations in adults. Acta Odontol Scand 62: 82-86. [Crossref]
- Manhart J, García-Godoy F, Hickel R (2002) Direct posterior restorations: clinical results and new developments. Dent Clin North Am 46: 303-339. [Crossref]
- Deligeorgi V, Mjör IA, Wilson NH (2001) An overview of reasons for the placement and replacement of restorations. Prim Dent Care 8: 5-11. [Crossref]
- Craig RG, Power JM (2002) Restorative Dental Materials, 11th edn. St Louis, MO: Mosby-Year Book, Inc 614-618.
- Wiegand A, Buchalla W, Attin T (2007) Review on fluoride-releasing restorative materials - Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23: 343-362. [Crossref]
- Osinaga PW, Grande RH, Ballester RY, Simionato MR, Delgado Rodrigues CR, et al. (2003) Zinc sulfate addition to glass-ionomer-based cements: influence on physical and antibacterial properties, zinc and fluoride release. Dent Mater 19: 212-217. [Crossref]
- Takahashi Y, Imazato S, Kaneshiro AV, Ebisu S, Frencken JE, et al. (2006) Antibacterial effects and physical properties of glass-ionomer cements containing chlorhexidine for the ART approach. Dent Mater 22: 647-52. [Crossref]
- Yamamoto K, Ohashi S, Aono M, Kokubo T, Yamada I, et al. (1996) Antibacterial activity of silver ions implanted in SiO2 filler on oral streptococci. Dent Mater 12: 227-229. [Crossref]
- Syafiuddin T, Hisamitsu H, Toko T, Igarashi T, Goto N, et al. (1997) In vitro inhibition of caries around a resin composite restoration containing antibacterial filler. Biomaterials 18: 1051-1057. [Crossref]
- Gottenbos B, van der Mei HC, Klatter F, Nieuwenhuis P, Busscher HJ (2002) In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials 23: 1417-1423. [Crossref]
- Thebault P, Taffin de Givenchy E, Levy R, Vandenberghe Y, Guittard F, et al. (2009) Preparation and antimicrobial behaviour of quaternary ammonium thiol derivatives able to be grafted on metal surfaces. Eur J Med Chem 44: 717-724. [Crossref]
- Imazato S, Russell RR, McCabe JF (1995) Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent 23: 177-181. [Crossref]
- Murata H (2007) Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 28: 4870-4879. [Crossref]
- Lu G, Wu D, Fu R (2007) Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React Funct Polym 67: 355-366.
- Lee SB, Koepsel RR, Morley SW, Matyjaszewski K, Sun Y, et al. (2004) Permanent, nonleaching antibacterial surfaces. 1. Synthesis by atom transfer radical polymerization. Biomacromolecules 5: 877-882. [Crossref]
- Li F, Chai ZG, Sun MN, Wang F, Ma S, et al. (2009) Anti-biofilm effect of dental adhesive with cationic monomer. J Dent Res 88: 372-376. [Crossref]
- Li F, Chen J, Chai Z, Zhang L, Xiao Y, et al. (2009) Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent 37: 289-296. [Crossref]
- Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI (2006) Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 27: 3995-4002. [Crossref]
- Chai Z, Li F, Fang M, Wang Y, Ma S, et al. (2011) The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J Oral Rehabil 38: 849-856. [Crossref]
- Ma S, Izutani N, Imazato S, Chen JH, Kiba W, et al. (2012) Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent Mater J 31:150-156. [Crossref]
- Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, et al. (2012) Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater 28: 561-572 [Crossref]
- Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, et al. (2012) Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J Dent Res 91: 460-466. [Crossref]
- Xie D, Weng Y, Guo X, Zhao J, Gregory RL, et al. (2011) Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater 27: 487-496. [Crossref]
- Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, et al. (2003) Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials 24: 3605-3609. [Crossref]
- Ebi N, Imazato S, Noiri Y, Ebisu S (2001) Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent Mater 17: 485-491. [Crossref]
- Jung JH, Pummangura S, Chaichantipyuth C, Patarapanich C, Fanwick PE, et al. (1990) New bioactive heptenes from melodorum fruticosum (annonaceae). Tetrahedron 46:5043-5054.
- Jones JB, Young JM (1968) Carcinogenicity of lactones. 3. The reactions of unsaturated gamma-lactones with L-cysteine. J Med Chem 11: 1176-1182. [Crossref]
- Lattmann E, Dunn S, Niamsanit S, Sattayasai N (2005) Synthesis and antibacterial activities of 5-hydroxy-4-amino-2(5H)-furanones. Bioorg Med Chem Lett 15: 919-921. [Crossref]
- Lattmann E, Coombs J, Hoffmann, HMR (1996) Paranofuranones via lewis acid mediated hetero-Diels-Alder reactions of 4-Furan-2(5H)-ones. A convergent route to the manoalide substructure. Synthesis 171-177.
- Martinelli D, Grossmann G, Séquin U, Brandl H, Bachofen R (2004) Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol 4: 25. [Crossref]
- Sung WS, Jung HJ, Lee IS, Lee DG (2006) Antimicrobial effect of furonoel against human pathogenic bacteria and fungi. J Microbiol Biotechnol 16: 349-354.
- Xie D, Weng Y, Zhao J (2009) Alternative methacrylate-tethering methods for resin-modified glass-ionomer cements. J Appl Polym Sci 111:869-875.
- Xie D, Park JG, Zhao J (2007) Synthesis and preparation of novel 4-arm star-shape poly(carboxylic acid)s for improved light-cured glass-ionomer cements. Dent Mater 23: 395-403. [Crossref]
- Wu W, Xie D, Puckett A, Mays J (2003) Synthesis and characterization of self-cured amino acid modified glass-ionomers. Eur Polym J 39: 959-968.
- Xie D, Chung I-D, Wu W, Lemons J, Puckett A, et al. (2004) An amino acid modified and non-HEMA containing glass-ionomer cement. Biomaterials 25(10): 1825-1830. [Crossref]
- Davidson CL, Mjor IA (1999) Advances in glass—ionomer cements. Chicago, IL: Quintessence Publishing Co.
- Zhao J, Xie D (2011) A novel hyperbranched poly(acrylic acid) for improved resin-modified glass-ionomer restoratives. Dent Mater 27: 478-486. [Crossref]
- Cattani-Lorente MA, Dupuis V, Moya F, Payan J, Meyer JM (1999) Comparative study of the physical properties of a polyacid-modified composite resin and a resin-modified glass ionomer cement. Dent Mater 15: 21-32. [Crossref]