Objectives: Fibroid non-enhancement is considered a relative contraindication to uterine artery embolization (UAE) for symptomatic fibroids. The purpose was to assess the impact of UAE on non-enhancing fibroids and to determine imaging predictors of fibroid shrinkage.
Materials and methods: Retrospective review of all women who underwent UAE for symptomatic fibroids (5/09-7/14) and had follow-up MRI 6 months after UAE were included. There were 59 fibroids (5, non-enhancing; 54, enhancing) among 18 women with a mean age of 46 (range 40-53) years. All fibroids were assessed for size, position, enhancement on subtraction images, and Apparent Diffusion Constant (ADC).
Results: Enhancing fibroids had an average decrease in diameter by 19± 3%, not significantly different that non-enhancing fibroids which decreased by 23± 6% (p=0.49). Multiple linear regression with percent change in fibroid diameter as the dependent variable and patient age, fibroid position, and pre-UAE fibroid diameter, enhancement, and ADC as independent variables, showed that ADC (p=0.04) and pre-UAE diameter (p=0.03) were the only significant independent variables.
Conclusion: Pre-UAE size and ADC, but not contrast enhancement, predicted fibroid diameter reduction. Enhancing and non-enhancing fibroids had similar size reduction after UAE. Non-enhancement should not be considered a contraindication to UAE.
uterine artery embolization, fibroid, enhancement, diffusivity
Uterine Artery Embolization (UAE) is a minimally invasive treatment with the potential to decrease fibroid size and reduce symptoms [1-3]. Volume reduction after embolization is associated with decreased fibroid-related symptoms, such as pain and dysmenorrhea [4,5]. Complete necrosis after UAE also predicts better long-term outcomes, with less pain and fibroid recurrence [4]. Through the use of Magnetic Resonance Imaging (MRI), fibroids are readily identified and monitored after UAE. Fibroids are typically hypointense on T2 and enhance following the intravenous administration of gadolinium due to ample blood supply [6].
Multiple papers have recommended that women with non-enhancing fibroids should be excluded from UAE due to anticipated minimal response in terms of size reduction [6-10], though there is a lack of studies directly comparing outcomes of enhancing vs non-enhancing fibroids. One retrospective study with 94 patients described the incidence of non-enhancing fibroids and recommended against their embolization, but did not report if the non-enhancing fibroids actually had poorer response [7]. For patients with unresectable hepatocellular carcinoma, chemoembolization is still performed for hypovascular tumors as there is evidence that a portion of these tumors do respond to embolization [11]. It is possible that non-enhancing fibroids respond similarly.
The purpose of the present study is to determine the response of necrotic fibroids to UAE and to identify whether pre-UAE enhancement or other factors are predictors of fibroid shrinkage.
Patients
This study was a retrospective review of all women who underwent UAE for symptomatic fibroids between May 2009 and July 2014 with follow-up MRI at 6 months after the procedure, identified through a search of the departmental picture archiving and communication system. Mean follow-up period was 7.4 months. A total of 59 fibroids (5, non-enhancing; 54, enhancing) were analyzed in 18 patients ranging between 40-53 years of age with a mean age of 46 years. Fibroids analyzed ranged in size from 1.6-18.2 cm with a mean diameter of 5.4 cm. Eighty-five other patients underwent UAE during this period, but did not have follow up imaging and were therefore excluded.
Uterine artery embolization
UAE was performed as previously described [12]. Briefly, procedures were performed with monitored anesthesia care. Access was obtained via the right common femoral artery, using a 5-Fr. introducer sheath (Cordis, Miami Lakes, FL); the anterior division of the internal iliac artery on each side was selected with a 5-French guiding Cobra catheter (Angiodynamics, Latham, NY). A 2.8-French ProGreat microcatheter (Terumo Interventional Systems, Somerset, NJ) was then directed into each uterine artery. Embolization was performed with 500-700-micron Embospheres (BioSphere Medical, Rockland, MA) until antegrade flow stasis. A completion peri-renal aortogram was performed to exclude ovarian artery supply to the uterus. Patients were admitted for at least one night of observation.
Image analysis
T1- and T2-weighted MR images were performed with and without gadolinium contrast enhancement within 2 months before UAE and at 6 months after UAE. For each fibroid, pre- and post-UAE size was determined by measuring the widest diameter on T2-weighted imaging. All fibroids measuring at least 2 cm were assessed for each patient. Fibroids were classified by position as either submucosal, intramural, or subserosal. T1-weighted signal intensity of each fibroid was recorded on dynamic contrast-enhanced subtraction MRI by placing a circular Region of Interest (ROI) over the axial or coronal slice with the largest cross-sectional area. Additionally, fibroids were characterized as non-enhancing or enhancing by subjective comparison of the fibroid to the myometrium on post-contrast images. Apparent Diffusion Constant (ADC) was determined for each pre-UAE fibroid with a corresponding ROI.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6. Comparisons were made using paired Student’s t-tests when variables were normally distributed and Mann-Whitney tests when variables were not normally distributed. A p<0.05 was considered to be statistically significant. Percent fibroid enhancement pre-UAE was calculated by dividing the fibroid signal intensity by myometrial signal intensity on contrast-enhanced T1W imaging. Multiple linear regression was used to determine if fibroid enhancement, ADC value, fibroid size, fibroid location (intramural, subserosal, submucosal), and patient age were independent predictors of fibroid shrinkage after UAE. Data is presented as mean± standard error of the mean.
A total of 59 fibroids were assessed, 39 (66%) of which were intramural. ADC was, on average 708.5 ± 74.2 × 10-6 mm2/s. Mean diameter was 53.6 ± 3.9 mm before UAE, and 44.0 ± 3.7 mm after UAE. Mean enhancement on subtraction MRI was 127.0 ± 15.5 SI before UAE and 3.9 ± 0.5 SI after UAE.
Overall, fibroids decreased in diameter by 20 ± 3%. Fifteen of the fibroids were non-enhancing. These fibroids decreased in diameter by 23 ± 6%, whereas enhancing fibroids decreased by 19 ± 3% (p=0.49) (Figure 1).
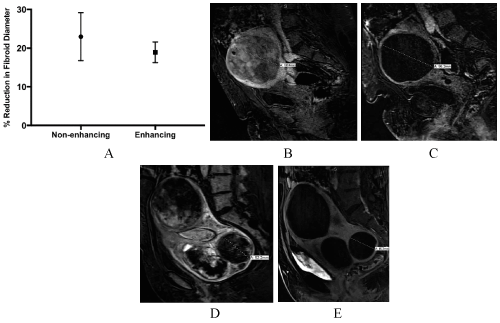
Figure 1. Shrinkage of enhancing and non-enhancing fibroids. (A) Non-enhancing and enhancing fibroids decreased in diameter by a similar percent after 4—6 months. T1-weight post-gadolinium MR images of an enhancing fibroid before (B) and after (C) UAE. A non-enhancing fibroid (D) demonstrated similar shrinkage (E).
Next, multiple linear regression with percent change in fibroid diameter as the dependent variable and patient age, fibroid location (subserosal, submucosal or intramural) and pre-UAE fibroid diameter, enhancement and ADC as independent variables was performed. Only ADC (p=0.04) and pre-UAE diameter (p=0.03) were significant independent variables; smaller diameter and higher ADC were associated with greater decreases in size. For example, fibroids with a pre-UAE diameter greater than the median (48.9 mm) decreased in diameter by 15% whereas fibroids with a pre-UAE diameter less than the median decreased in diameter by 25% (p=0.05). In contrast, pre-UAE enhancement was not an independent predictor of post-UAE size diminution (p=0.31).
This study demonstrates that non-enhancing fibroids have similar decrease in size compared to enhancing fibroids after UAE. Furthermore, contrast enhancement on MRI did not predict changes in fibroid size, suggesting that patients with minimally enhancing and non-enhancing fibroids may still benefit from UAE. Currently, there is conflicting data in the literature in regards to fibroid enhancement as a predictor of UAE response. For instance, one study of 28 fibroids reported that avidly enhancing fibroids had greater volume reduction [8]. Other studies reported to the contrary that greater enhancement pre-UAE does not necessarily predict better response [13-15]. One study did not find a greater volume reduction 4 months post-UAE between 14 fibroids that were well perfused compared to 21 fibroids that were not well perfused [13]. More recently, a study of 182 patients reported that low vascularity might predict a more favorable outcome in patients treated with embolization; it was found that patients with lower vascularity fibroids had lower incidences of recurrences compared to patients with higher vascularity fibroids [14]. Additionally, avidly enhancing fibroids rapidly decreased in size shortly after embolization but plateaued after 6 months, as opposed to less enhancing fibroids that continued to shrink after 6 months [14]. This may explain why previous studies with shorter length to follow-up reported greater volume reduction in enhancing fibroids [8,9].
Our study adds to this literature by showing that non-enhancing and presumably necrotic fibroids respond to UAE, despite their relative avascularity. Therefore, women with necrotic fibroids should not be excluded from treatment. Though one may assume that embolization should be more effective for fibroids of greater vascularity, fibroids have particularly abnormal angiogenesis and vascularization patterns that are not completely understood. The avascular and hypoxic environment of fibroids is paradoxically associated with more pro-angiogenic factors [16].
Our findings also suggest that ADC is an independent predictor of fibroid shrinkage. Fibroids with higher ADC values pre-UAE demonstrated greater shrinkage, which has been reported in other retrospective and prospective investigations [9,15,17]. It is unclear what the degree of diffusion restriction of water molecules signifies, but ADC provides some information about the molecular and/or cellular composition of masses including fibroids, and it may be useful in pre-UAE patient selection. We also found that smaller fibroid size predicted greater reduction after UAE, which is in contrast to other studies that showed either no correlation between fibroid size and volume reduction, or better response in larger fibroids [8,18].
The primary limitations of this study pertain to its single-center retrospective nature. Our primary marker of UAE response is restricted to fibroid size instead of clinical outcomes due to limited data availability in our medical database. The length of follow-up is also limited due to the practice of obtaining MRI imaging 6 months after embolization without the need for longer-term imaging. A longer term prospective study with a larger sample size and clinical correlates could therefore increase the accuracy of our evaluations and help prove that patients with minimally or non-enhancing fibroids can still benefit from embolization. In addition to fibroid size reduction on imaging, clinical markers such as bleeding, pain, and recurrence could also be measured. Fibroid size is a more objective marker, reflects data easier to collect, and generally associated with symptom improvement, but clinical markers will more accurately predict response [4,5].
In conclusion, non-enhancing fibroids respond to UAE, particularly in regard to size reduction. In addition, MRI characteristics such as ADC values may better predict response to UAE. Especially for women who either do not desire surgery or want to preserve fertility, UAE may be the best option and it is important not to withhold therapy without clear evidence of its inefficacy, in the setting of non-enhancement. These results suggest that physicians should reconsider excluding women with non-enhancing fibroids, as these patients may also benefit from embolization.
- Goodwin SC, McLucas B, Lee M, Chen G, Perrella R, et al. (1999) Uterine artery embolization for the treatment of uterine leiomyomata midterm results. J Vasc Interv Radiol 10: 1159-65. [Crossref]
- Spies JB1, Scialli AR, Jha RC, Imaoka I, Ascher SM, et al. (1999) Initial results from uterine fibroid embolization for symptomatic leiomyomata. J Vasc Interv Radiol 10: 1149-1157. [Crossref]
- Hutchins FL Jr, Worthington-Kirsch R, Berkowitz RP (1999) Selective uterine artery embolization as primary treatment for symptomatic leiomyomata uteri. J Am Assoc Gynecol Laparosc 6: 279-84. [Crossref]
- Spies JB, Roth AR, Jha RC, Gomez-Jorge J, Levy EB et al, (2002) Leiomyomata treated with uterine artery embolization: factors associated with successful symptom and imaging outcome. Radiology 222: 45-52. [Crossref]
- Toor SS, Tan KT, Simons ME, Rajan DK, Beecroft JR et al. (2008) Clinical failure after uterine artery embolization: evaluation of patient and MR imaging characteristics. J Vasc Interv Radiol 19: 662-7. [Crossref]
- Kirby JM, Burrows D, Haider E, Maizlin Z, Midia M (2011) Utility of MRI before and after uterine fibroid embolization: why to do it and what to look for. Cardiovasc Intervent Radiol 34: 705-716. [Crossref]
- Nikolaidis P, Siddiqi AJ, Carr JC, Vogelzang RL, Miller FH, et al. (2005) Incidence of nonviable leiomyomas on contrast material-enhanced pelvic MR imaging in patients referred for uterine artery embolization. J Vasc Interv Radiol 16: 1465-71. [Crossref]
- Harman M, Zeteroğlu S, Arslan H, Sengül M, Etlik O, et al. (2006) Predictive value of magnetic resonance imaging signal and contrast-enhancement characteristics on post-embolization volume reduction of uterine fibroids. Acta Radiol 47: 427-35. [Crossref]
- Han SC, Kim MD, Jung DC, Lee M, Lee MS, et al. (2013) Degeneration of leiomyoma in patients referred for uterine fibroid embolization: incidence, imaging features and clinical characteristics. Yonsei Med J 54: 215-9. [Crossref]
- Keeling AN, JF Reidy (2007) Imaging and treatment of uterine fibroids, including the role of uterine artery embolization. Imaging 19: 374-384.
- Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, et al. (2003) Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer 97: 1042-1050. [Crossref]
- Worthington-Kirsch RL, Popky GL, Hutchins FL Jr (1998) Uterine arterial embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology 208: 625-9. [Crossref]
- deSouza NM, Williams AD (2002) Uterine arterial embolization for leiomyomas: perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology 222: 367-74. [Crossref]
- Tang Y, Chen C, Duan H, Ma B, Liu P, et al. (2016) Low vascularity predicts favourable outcomes in leiomyoma patients treated with uterine artery embolization. Eur Radiol 26: 3571-9 [Crossref]
- Hecht EM, Do RK, Kang SK, Bennett GL, Babb JS, et al. (2011) Diffusion-weighted imaging for prediction of volumetric response of leiomyomas following uterine artery embolization: A preliminary study. Journal of Magnetic Resonance Imaging 33: 641-646. [Crossref]
- Tal R, JH Segars (2014) The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update 20: 194-216. [Crossref]
- Ananthakrishnan, G, G Macnaught, L Hinksman, H Gilmour, K P Forbes et al. (2012) Diffusion-weighted imaging in uterine artery embolisation: do findings correlate with contrast enhancement and volume reduction? Br J Radiol 85: e1046-50. [Crossref]
- Burn PR, McCall JM, Chinn RJ, Vashisht A, Smith JR, et al. (2000) Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology 214: 729-34. [Crossref]

